Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF
- PMID: 11724822
- PMCID: PMC2150867
- DOI: 10.1083/jcb.200106139
Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF
Abstract
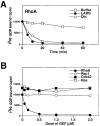
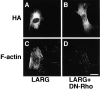
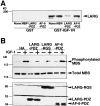
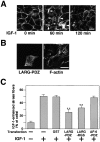
Insulin-like growth factor (IGF)-1 plays crucial roles in growth control and rearrangements of the cytoskeleton. IGF-1 binds to the IGF-1 receptor and thereby induces the autophosphorylation of this receptor at its tyrosine residues. The phosphorylation of the IGF-1 receptor is thought to initiate a cascade of events. Although various signaling molecules have been identified, they appear to interact with the tyrosine-phosphorylated IGF-1 receptor. Here, we identified leukemia-associated Rho guanine nucleotide exchange factor (GEF) (LARG), which contains the PSD-95/Dlg/ZO-1 (PDZ), regulator of G protein signaling (RGS), Dbl homology, and pleckstrin homology domains, as a nonphosphorylated IGF-1 receptor-interacting molecule. LARG formed a complex with the IGF-1 receptor in vivo, and the PDZ domain of LARG interacted directly with the COOH-terminal domain of IGF-1 receptor in vitro. LARG had an exchange activity for Rho in vitro and induced the formation of stress fibers in NIH 3T3 fibroblasts. When MDCKII epithelial cells were treated with IGF-1, Rho and its effector Rho-associated kinase (Rho-kinase) were activated and actin stress fibers were enhanced. Furthermore, the IGF-1-induced Rho-kinase activation and the enhancement of stress fibers were inhibited by ectopic expression of the PDZ and RGS domains of LARG. Taken together, these results indicate that IGF-1 activates the Rho/Rho-kinase pathway via a LARG/IGF-1 receptor complex and thereby regulates cytoskeletal rearrangements.
Figures






Similar articles
-
Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential.Oncogene. 2004 Jan 8;23(1):233-40. doi: 10.1038/sj.onc.1207012. Oncogene. 2004. PMID: 14712228
-
Characterization of leukemia-associated Rho guanine nucleotide exchange factor (LARG) expression during murine development.Cell Tissue Res. 2003 Dec;314(3):361-6. doi: 10.1007/s00441-003-0802-5. Epub 2003 Sep 26. Cell Tissue Res. 2003. PMID: 14513355
-
Galpha 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF.Proc Natl Acad Sci U S A. 2003 Jan 21;100(2):733-8. doi: 10.1073/pnas.0234057100. Epub 2003 Jan 6. Proc Natl Acad Sci U S A. 2003. PMID: 12515866 Free PMC article.
-
Pleckstrin homology domains and phospholipid-induced cytoskeletal reorganization.Thromb Haemost. 1999 Aug;82(2):399-406. Thromb Haemost. 1999. PMID: 10605730 Review.
-
RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho?Oncogene. 2001 Mar 26;20(13):1661-8. doi: 10.1038/sj.onc.1204182. Oncogene. 2001. PMID: 11313914 Review. No abstract available.
Cited by
-
Identification of a novel sequence in PDZ-RhoGEF that mediates interaction with the actin cytoskeleton.Mol Biol Cell. 2004 Apr;15(4):1760-75. doi: 10.1091/mbc.e03-07-0527. Epub 2004 Jan 23. Mol Biol Cell. 2004. PMID: 14742719 Free PMC article.
-
Signalling mechanisms of RhoGTPase regulation by the heterotrimeric G proteins G12 and G13.J Biochem. 2011 Oct;150(4):357-69. doi: 10.1093/jb/mvr105. Epub 2011 Aug 26. J Biochem. 2011. PMID: 21873336 Free PMC article. Review.
-
Aging and gastrointestinal neuromuscular function: insights from within and outside the gut.Neurogastroenterol Motil. 2011 Jun;23(6):490-501. doi: 10.1111/j.1365-2982.2011.01678.x. Epub 2011 Feb 15. Neurogastroenterol Motil. 2011. PMID: 21320236 Free PMC article. Review. No abstract available.
-
Critical role for Kalirin in nerve growth factor signaling through TrkA.Mol Cell Biol. 2005 Jun;25(12):5106-18. doi: 10.1128/MCB.25.12.5106-5118.2005. Mol Cell Biol. 2005. PMID: 15923627 Free PMC article.
-
Stromal-derived IGF2 promotes colon cancer progression via paracrine and autocrine mechanisms.Oncogene. 2017 Sep 21;36(38):5341-5355. doi: 10.1038/onc.2017.116. Epub 2017 May 22. Oncogene. 2017. PMID: 28534511
References
-
- Abe, K., K.L. Rossman, B. Liu, K.D. Ritola, D. Chiang, S.L. Campbell, K. Burridge, and C.J. Der. 2000. Vav2 is an activator of Cdc42, Rac1, and RhoA. J. Biol. Chem. 275:10141–10149. - PubMed
-
- Blangy, A., E. Vignal, S. Schmidt, A. Debant, C. Gauthier-Rouviere, and P. Fort. 2000. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J. Cell Sci. 113:729–739. - PubMed
-
- Cerione, R.A., and Y. Zheng. 1996. The Dbl family of oncogenes. Curr. Opin. Cell Biol. 8:216–222. - PubMed
-
- Cheng, H.L., M.L. Steinway, J.W. Russell, and E.L. Feldman. 2000. GTPases and phosphatidylinositol 3-kinase are critical for insulin-like growth factor-I-mediated Schwann cell motility. J. Biol. Chem. 275:27197–27204. - PubMed
-
- Crespo, P., K.E. Schuebel, A.A. Ostrom, J.S. Gutkind, and X.R. Bustelo. 1997. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 385:169–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

