Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay
- PMID: 11724856
- PMCID: PMC88560
- DOI: 10.1128/JCM.39.12.4426-4432.2001
Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay
Abstract
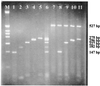
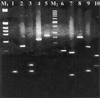
A sensitive multiplex PCR assay for single-tube amplification that detects simultaneous herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), varicella-zoster virus (VZV), human cytomegalovirus (CMV), and Epstein-Barr virus (EBV) is reported with particular emphasis on how the method was optimized and carried out and its sensitivity was compared to previously described assays. The assay has been used on a limited number of clinical samples and must be thoroughly evaluated in the clinical context. A total of 86 cerebrospinal fluid (CSF) specimens from patients which had the clinical symptoms of encephalitis, meningitis or meningoencephalitis were included in this study. The sensitivity of the multiplex PCR was determined to be 0.01 and 0.03 50% tissue culture infective doses/the reciprocal of the highest dilution positive by PCR for HSV-1 and HSV-2 respectively, whereas for VZV, CMV and EBV, 14, 18, and 160 ag of genomic DNA were detected corresponding to 48, 66, and 840 genome copies respectively. Overall, 9 (10.3%) of the CSF samples tested were positive in the multiplex PCR. HSV-1 was detected in three patients (3.5%) with encephalitis, VZV was detected in four patients (4.6%) with meningitis, HSV-2 was detected in one neonate (1.16%), and CMV was also detected in one neonate (1.16%). None of the samples tested was positive for the EBV genome. None of the nine positive CSF samples presented herpesvirus coinfection in the central nervous system. Failure of DNA extraction or failure to remove any inhibitors of DNA amplification from CSF samples was avoided by the inclusion in the present multiplex PCR assay of alpha-tubulin primers. The present multiplex PCR assay detects simultaneously five different herpesviruses and sample suitability for PCR in a single amplification round of 40 cycles with an excellent sensitivity and can, therefore, provide an early, rapid, reliable noninvasive diagnostic tool allowing the application of antiviral therapy on the basis of a specific viral diagnosis. The results of this preliminary study should prompt a more exhaustive analysis of the clinical value of the present multiplex PCR assay.
Figures
Similar articles
-
Single tube multiplex real-time PCR for the rapid detection of herpesvirus infections of the central nervous system.Mol Cell Probes. 2011 Apr-Jun;25(2-3):114-20. doi: 10.1016/j.mcp.2011.03.004. Epub 2011 Apr 3. Mol Cell Probes. 2011. PMID: 21466846
-
Comparison of nested PCR and real time PCR of Herpesvirus infections of central nervous system in HIV patients.BMC Infect Dis. 2004 Nov 30;4:55. doi: 10.1186/1471-2334-4-55. BMC Infect Dis. 2004. PMID: 15571633 Free PMC article.
-
Automated detection of five human herpes virus DNAs by a set of LightCycler PCRs complemented with a single multiple internal control.J Clin Virol. 2004 Mar;29(3):171-8. doi: 10.1016/S1386-6532(03)00121-5. J Clin Virol. 2004. PMID: 14962786
-
Diagnosis of herpesvirus infections of the central nervous system.Herpes. 2004 Jun;11 Suppl 2:48A-56A. Herpes. 2004. PMID: 15319090 Review.
-
Use of PCR for the diagnosis of herpesvirus infections of the central nervous system.J Clin Virol. 2002 Jul;25 Suppl 1:S5-11. doi: 10.1016/s1386-6532(02)00028-8. J Clin Virol. 2002. PMID: 12091076 Review.
Cited by
-
Multiplex PCR: rapid DNA cycling in a conventional thermal cycler.J Clin Lab Anal. 2003;17(4):108-12. doi: 10.1002/jcla.10082. J Clin Lab Anal. 2003. PMID: 12784258 Free PMC article.
-
A role for arrays in clinical virology: fact or fiction?J Clin Virol. 2004 Jan;29(1):2-12. doi: 10.1016/j.jcv.2003.08.002. J Clin Virol. 2004. PMID: 14675863 Free PMC article. Review.
-
Clinico-epidemiological Study of Viral Acute Encephalitis Syndrome Cases and Comparison to Nonviral Cases in Children from Eastern India.J Glob Infect Dis. 2019 Jan-Mar;11(1):7-12. doi: 10.4103/jgid.jgid_26_18. J Glob Infect Dis. 2019. PMID: 30814829 Free PMC article.
-
Divergent cerebrospinal fluid cytokine network induced by non-viral and different viral infections on the central nervous system.BMC Infect Dis. 2015 Aug 19;15:345. doi: 10.1186/s12879-015-1035-4. BMC Infect Dis. 2015. PMID: 26286516 Free PMC article.
-
Structural and functional characterization of a multifunctional alanine-rich peptide analogue from Pleuronectes americanus.PLoS One. 2012;7(10):e47047. doi: 10.1371/journal.pone.0047047. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056574 Free PMC article.
References
-
- Aslanzadeh J, Osmon D R, Wilhelm M P, Espy M J, Smith T F. A prospective study of the polymerase chain reaction for selection of herpes simplex virus in cerebrospinal fluid submitted to the clinical virology laboratory. Mol Cell Probes. 1992;6:367–373. - PubMed
-
- Aurelius E. Herpes simplex encephalitis—early diagnosis and immune activation in the acute stage and during long-term follow up. Scand J Infect Dis Suppl. 1993;89:3–62. - PubMed
-
- Bos C A, Olding-Stenkvist E, Wilterdink J B, Scheffer A. Detection of viral antigens in cerebrospinal fluid of patients with herpes simplex virus encephalitis. J Med Virol. 1987;21:169–178. - PubMed
-
- Casas I, Powell L, Klapper P E, Cleator G M. New method for the extraction of viral RNA and DNA from cerebrospinal fluid for use in the polymerase chain reaction assay. J Virol Methods. 1995;53:25–36. - PubMed
-
- Casas I, Tenorio A, de Ory F, Lozano A, Echevarria J M. Detection of both herpes simplex and varicella-zoster viruses in cerebrospinal fluid from patients with encephalitis. J Med Virol. 1996;50:82–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

