Critical involvement of a carbamylated lysine in catalytic function of class D beta-lactamases
- PMID: 11724923
- PMCID: PMC64673
- DOI: 10.1073/pnas.241442898
Critical involvement of a carbamylated lysine in catalytic function of class D beta-lactamases
Abstract
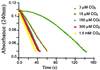
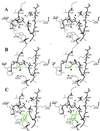
beta-Lactamases are the resistance enzymes for beta-lactam antibiotics, of which four classes are known. beta-lactamases hydrolyze the beta-lactam moieties of these antibiotics, rendering them inactive. It is shown herein that the class D OXA-10 beta-lactamase depends critically on an unusual carbamylated lysine as the basic residue for both the enzyme acylation and deacylation steps of catalysis. The formation of carbamylated lysine is reversible. Evidence is presented that this enzyme is dimeric and carbamylated in living bacteria. High-resolution x-ray structures for the native enzyme were determined at pH values of 6.0, 6.5, 7.5, and 8.5. Two dimers are present per asymmetric unit. One monomer in each dimer was carbamylated at pH 6.0, whereas all four monomers were fully carbamylated at pH 8.5. At the intermediate pH values, one monomer of each dimer was carbamylated, and the other showed a mixture of carbamylated and non-carbamylated lysines. It would appear that, as the pH increased for the sample, additional lysines were "titrated" by carbamylation. A handful of carbamylated lysines are known from protein crystallographic data, all of which have been attributed roles in structural stabilization (mostly as metal ligands) of the proteins. This paper reports a previously unrecognized role for a noncoordinated carbamylate lysine as a basic residue involved in mechanistic reactions of an enzyme, which indicates another means for expansion of the catalytic capabilities of the amino acids in nature beyond the 20 common amino acids in development of biological catalysts.
Figures


Similar articles
-
Crystal structure of the narrow-spectrum OXA-46 class D beta-lactamase: relationship between active-site lysine carbamylation and inhibition by polycarboxylates.Antimicrob Agents Chemother. 2010 May;54(5):2167-74. doi: 10.1128/AAC.01517-09. Epub 2010 Feb 9. Antimicrob Agents Chemother. 2010. PMID: 20145076 Free PMC article.
-
Critical role of tryptophan 154 for the activity and stability of class D beta-lactamases.Biochemistry. 2009 Dec 1;48(47):11252-63. doi: 10.1021/bi901548c. Biochemistry. 2009. PMID: 19860471
-
Crystal structures of the class D beta-lactamase OXA-13 in the native form and in complex with meropenem.J Mol Biol. 2001 Jul 20;310(4):859-74. doi: 10.1006/jmbi.2001.4805. J Mol Biol. 2001. PMID: 11453693
-
Substrate deacylation mechanisms of serine-beta-lactamases.Biol Pharm Bull. 2006 Nov;29(11):2151-9. doi: 10.1248/bpb.29.2151. Biol Pharm Bull. 2006. PMID: 17077507 Review.
-
The other kind of biological NMR--studies of enzyme-substrate interactions.Neurochem Res. 1996 Sep;21(9):1117-24. doi: 10.1007/BF02532422. Neurochem Res. 1996. PMID: 8897475 Review.
Cited by
-
An amino acid position at crossroads of evolution of protein function: antibiotic sensor domain of BlaR1 protein from Staphylococcus aureus versus clasS D β-lactamases.J Biol Chem. 2012 Mar 9;287(11):8232-41. doi: 10.1074/jbc.M111.333179. Epub 2012 Jan 18. J Biol Chem. 2012. PMID: 22262858 Free PMC article.
-
Clinical Variants of the Native Class D β-Lactamase of Acinetobacter baumannii Pose an Emerging Threat through Increased Hydrolytic Activity against Carbapenems.Antimicrob Agents Chemother. 2016 Sep 23;60(10):6155-64. doi: 10.1128/AAC.01277-16. Print 2016 Oct. Antimicrob Agents Chemother. 2016. PMID: 27480863 Free PMC article.
-
VNRX-5133 (Taniborbactam), a Broad-Spectrum Inhibitor of Serine- and Metallo-β-Lactamases, Restores Activity of Cefepime in Enterobacterales and Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e01963-19. doi: 10.1128/AAC.01963-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31871094 Free PMC article.
-
Biosynthesis of the proteasome inhibitor syringolin A: the ureido group joining two amino acids originates from bicarbonate.BMC Biochem. 2009 Oct 28;10:26. doi: 10.1186/1471-2091-10-26. BMC Biochem. 2009. PMID: 19863801 Free PMC article.
-
Methylation and carbamylation of human gamma-crystallins.Protein Sci. 2003 Aug;12(8):1762-74. doi: 10.1110/ps.0305403. Protein Sci. 2003. PMID: 12876325 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

