Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease
- PMID: 11724929
- PMCID: PMC64739
- DOI: 10.1073/pnas.251341998
Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease
Abstract
Parkinson's disease is a chronic neurodegenerative disorder characterized by the loss of dopamine neurons in the substantia nigra, decreased striatal dopamine levels, and consequent extrapyramidal motor dysfunction. We now report that minocycline, a semisynthetic tetracycline, recently shown to have neuroprotective effects in animal models of stroke/ischemic injury and Huntington's disease, prevents nigrostriatal dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Minocycline treatment also blocked dopamine depletion in the striatum as well as in the nucleus accumbens after MPTP administration. The neuroprotective effect of minocycline is associated with marked reductions in inducible NO synthase (iNOS) and caspase 1 expression. In vitro studies using primary cultures of mesencephalic and cerebellar granule neurons (CGN) and/or glia demonstrate that minocycline inhibits both 1-methyl-4-phenylpyridinium (MPP(+))-mediated iNOS expression and NO-induced neurotoxicity, but MPP(+)-induced neurotoxicity is inhibited only in the presence of glia. Further, minocycline also inhibits NO-induced phosphorylation of p38 mitogen-activated protein kinase (MAPK) in CGN and the p38 MAPK inhibitor, SB203580, blocks NO toxicity of CGN. Our results suggest that minocycline blocks MPTP neurotoxicity in vivo by indirectly inhibiting MPTP/MPP(+)-induced glial iNOS expression and/or directly inhibiting NO-induced neurotoxicity, most likely by inhibiting the phosphorylation of p38 MAPK. Thus, NO appears to play an important role in MPTP neurotoxicity. Neuroprotective tetracyclines may be effective in preventing or slowing the progression of Parkinson's and other neurodegenerative diseases.
Figures

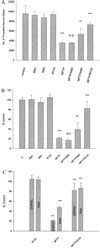



References
-
- Oertel W H, Quinn N P. In: Neurological Disorders: Course and Treatment. Brandt T, Diener H C, Caplan L R, Kennard C, Dichgans J, editors. San Diego: Academic; 1996. pp. 715–772.
-
- Quinn N P. Neurology. 1998;51:S25–S29. - PubMed
-
- Tolwani R J, Jakowec M W, Petzinger G M, Green S, Waggie K. Lab Anim Sci. 1999;49:363–371. - PubMed
-
- Langston J W, Ballard J W, Tetrud J W, Irwin I. Science. 1983;219:979–980. - PubMed
-
- Uhl G R, Javitch J A, Snyder S H. Lancet. 1985;1:956–957. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

