The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum
- PMID: 11724934
- PMCID: PMC64697
- DOI: 10.1073/pnas.251401598
The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum
Abstract
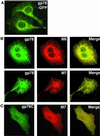
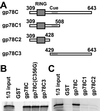
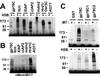
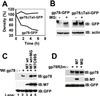
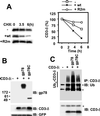
gp78, also known as the tumor autocrine motility factor receptor, is a transmembrane protein whose expression is correlated with tumor metastasis. We establish that gp78 is a RING finger-dependent ubiquitin protein ligase (E3) of the endoplasmic reticulum (ER). Consistent with this, gp78 specifically recruits MmUBC7, a ubiquitin-conjugating enzyme (E2) implicated in ER-associated degradation (ERAD), through a region distinct from the RING finger. gp78 can target itself for proteasomal degradation in a RING finger- and MmUBC7-dependent manner. Importantly, gp78 can also mediate degradation of CD3-delta, a well-characterized ERAD substrate. In contrast, gp78 lacking an intact RING finger or its multiple membrane-spanning domains stabilizes CD3-delta. gp78 has thus been found to be an example of a mammalian cellular E3 intrinsic to the ER, suggesting a potential link between ubiquitylation, ERAD, and metastasis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

