Genetically engineered macrophages expressing IFN-gamma restore alveolar immune function in scid mice
- PMID: 11724936
- PMCID: PMC64726
- DOI: 10.1073/pnas.251451498
Genetically engineered macrophages expressing IFN-gamma restore alveolar immune function in scid mice
Abstract
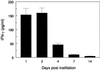
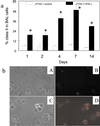
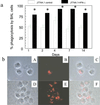
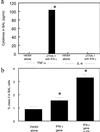
Reversal of immunodeficiency in the lung by gene therapy is limited in part by the difficulty of transfecting lung cells in vivo. Many options exist for successfully transfecting cells in vitro, but they are not easily adapted to the in vivo condition. To overcome this limitation, we transduced macrophages in vitro with the murine IFN-gamma (mIFN-gamma) gene and intratracheally delivered the macrophages to express mIFN-gamma in vivo. A recombinant retroviral vector pSF91 system was modified to encode mIFN-gamma and enhanced green fluorescent protein (EGFP). A murine macrophage cell line J774A.1 transduced with the retroviral supernatant increased secretion from undetectable levels to 131.6 +/- 4.2 microg/ml mIFN-gamma at 24 h in vitro. The mIFN-gamma-producing macrophages were intratracheally instilled into mechanically ventilated scid mice. mIFN-gamma levels in the bronchoalveolar lavage increased from undetectable levels at baseline to 158.8 +/- 5.1 pg/ml at 48 h (P < 0.001). Analysis of the lavaged cells for EGFP expression revealed that EGFP expression was directly proportional to the number of transduced macrophages instilled into the lung. Immune function was partially restored in the alveolar spaces of scid mice with evidence of enhanced MHC class II antigen expression and increased phagocytosis (P < 0.05). Tumor necrosis factor alpha was increased from undetectable at baseline to 103.5 +/- 11.4 pg/ml. In contrast, i.p. administration of the engineered macrophages did not enhance IFN-gamma levels in the lung. Our study suggests airway delivery of genetically engineered macrophages expressing mIFN-gamma gene can partially restore significant immune activity in the lungs of immunodeficient mice.
Figures



Similar articles
-
Airway delivery of interferon-γ overexpressing macrophages confers resistance to Mycobacterium avium infection in SCID mice.Physiol Rep. 2016 Nov;4(21):e13008. doi: 10.14814/phy2.13008. Epub 2016 Nov 17. Physiol Rep. 2016. PMID: 27856731 Free PMC article.
-
Expression of interferon-gamma by stromal cells inhibits murine long-term repopulating hematopoietic stem cell activity.Exp Hematol. 1999 May;27(5):895-903. doi: 10.1016/s0301-472x(99)00009-0. Exp Hematol. 1999. PMID: 10340406
-
Passive transfer of interferon-γ over-expressing macrophages enhances resistance of SCID mice to Mycobacterium tuberculosis infection.Cytokine. 2017 Jul;95:70-79. doi: 10.1016/j.cyto.2017.02.009. Epub 2017 Feb 24. Cytokine. 2017. PMID: 28237876
-
Enhanced protection against fatal mycobacterial infection in SCID beige mice by reshaping innate immunity with IFN-gamma transgene.J Immunol. 2001 Jul 1;167(1):375-83. doi: 10.4049/jimmunol.167.1.375. J Immunol. 2001. PMID: 11418673
-
A novel approach to restore lung immunity during systemic immunosuppression.Trans Am Clin Climatol Assoc. 2005;116:221-6; discussion 226-7. Trans Am Clin Climatol Assoc. 2005. PMID: 16555616 Free PMC article.
Cited by
-
Elevated inflammatory response in caveolin-1-deficient mice with Pseudomonas aeruginosa infection is mediated by STAT3 protein and nuclear factor kappaB (NF-kappaB).J Biol Chem. 2011 Jun 17;286(24):21814-25. doi: 10.1074/jbc.M111.237628. Epub 2011 Apr 22. J Biol Chem. 2011. PMID: 21515682 Free PMC article.
-
MEG3-4 is a miRNA decoy that regulates IL-1β abundance to initiate and then limit inflammation to prevent sepsis during lung infection.Sci Signal. 2018 Jun 26;11(536):eaao2387. doi: 10.1126/scisignal.aao2387. Sci Signal. 2018. PMID: 29945883 Free PMC article.
-
Human 8-oxoguanine DNA glycosylase increases resistance to hyperoxic cytotoxicity in lung epithelial cells and involvement with altered MAPK activity.Cell Death Differ. 2006 Feb;13(2):311-23. doi: 10.1038/sj.cdd.4401736. Cell Death Differ. 2006. PMID: 16052235 Free PMC article.
-
Structural and Molecular Mechanism of CdpR Involved in Quorum-Sensing and Bacterial Virulence in Pseudomonas aeruginosa.PLoS Biol. 2016 Apr 27;14(4):e1002449. doi: 10.1371/journal.pbio.1002449. eCollection 2016 Apr. PLoS Biol. 2016. PMID: 27119725 Free PMC article.
-
Euthanasia method for mice in rapid time-course pulmonary pharmacokinetic studies.J Am Assoc Lab Anim Sci. 2009 Sep;48(5):506-11. J Am Assoc Lab Anim Sci. 2009. PMID: 19807971 Free PMC article.
References
-
- Hunninghake G W, Crystal R G. N Engl J Med. 1981;305:429–434. - PubMed
-
- Limper A H, Offord K P, Smith T F, Martin II W J. Am Rev Respir Dis. 1989;140:1024–1209. - PubMed
-
- Pifer L L, Hughes T, Stagno S, Woods D. Pediatrics. 1978;62:35–41. - PubMed
-
- Reynolds H Y. Clin Chest Med. 1987;8:339–559. - PubMed
-
- Hunninghake G W, Fauci A S. J Immunol. 1977;118:146–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

