Activation of the Mason-Pfizer monkey virus protease within immature capsids in vitro
- PMID: 11724937
- PMCID: PMC64733
- DOI: 10.1073/pnas.251460998
Activation of the Mason-Pfizer monkey virus protease within immature capsids in vitro
Abstract
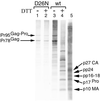
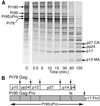
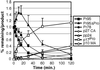
For all retroviruses, the completion of the viral budding process correlates with the activation of the viral protease by an unknown mechanism, and, as the structural (Gag) polyproteins are cleaved by the viral protease, maturation of the immature virus-like particle into an infectious virion. Unlike most retroviruses, the Mason-Pfizer monkey virus Gag polyproteins assemble into immature capsids within the cytoplasm of the cell before the viral budding event. The results reported here describe a unique experimental system in which Mason-Pfizer monkey virus immature capsids are removed from the cell, and the protease is activated in vitro by the addition of a reducing agent. The cleavage of the protease from the precursor form is a primary event, which proceeds with a half time of 14 min, and is followed by authentic processing of the Gag polyproteins. Activity of the viral protease in vitro depends on pH, with an increase in catalytic rates at acidic and neutral pH. The initiation of protease activity within immature capsids in vitro demonstrates that viral protease activity is sensitive to oxidation-reduction conditions, and that the viral protease can be activated in the absence of viral budding.
Figures
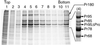


Similar articles
-
A proline-rich motif (PPPY) in the Gag polyprotein of Mason-Pfizer monkey virus plays a maturation-independent role in virion release.J Virol. 1998 May;72(5):4095-103. doi: 10.1128/JVI.72.5.4095-4103.1998. J Virol. 1998. PMID: 9557699 Free PMC article.
-
Specific in vitro cleavage of Mason-Pfizer monkey virus capsid protein: evidence for a potential role of retroviral protease in early stages of infection.Virology. 2003 Jun 5;310(2):310-8. doi: 10.1016/s0042-6822(03)00128-4. Virology. 2003. PMID: 12781718
-
The role of the S-S bridge in retroviral protease function and virion maturation.J Mol Biol. 2007 Feb 2;365(5):1493-504. doi: 10.1016/j.jmb.2006.11.005. Epub 2006 Nov 6. J Mol Biol. 2007. PMID: 17140600
-
Activation mechanism of pepsinogen as compared to the processing of HIV protease gag-pol precursor protein.Adv Exp Med Biol. 1998;436:245-52. doi: 10.1007/978-1-4615-5373-1_34. Adv Exp Med Biol. 1998. PMID: 9561226 Review. No abstract available.
-
Interaction of Mason-Pfizer monkey virus matrix protein with plasma membrane.Front Microbiol. 2014 Jan 21;4:423. doi: 10.3389/fmicb.2013.00423. eCollection 2013. Front Microbiol. 2014. PMID: 24478762 Free PMC article. Review.
Cited by
-
The effect of point mutations within the N-terminal domain of Mason-Pfizer monkey virus capsid protein on virus core assembly and infectivity.Virology. 2008 Oct 10;380(1):157-63. doi: 10.1016/j.virol.2008.07.021. Epub 2008 Aug 27. Virology. 2008. PMID: 18755489 Free PMC article.
-
High-resolution structure of a retroviral protease folded as a monomer.Acta Crystallogr D Biol Crystallogr. 2011 Nov;67(Pt 11):907-14. doi: 10.1107/S0907444911035943. Epub 2011 Oct 19. Acta Crystallogr D Biol Crystallogr. 2011. PMID: 22101816 Free PMC article.
-
Reversible oxidative modification as a mechanism for regulating retroviral protease dimerization and activation.J Virol. 2003 Mar;77(5):3319-25. doi: 10.1128/jvi.77.5.3319-3325.2003. J Virol. 2003. PMID: 12584357 Free PMC article.
-
Regulation of the Dimerization and Activity of SARS-CoV-2 Main Protease through Reversible Glutathionylation of Cysteine 300.bioRxiv [Preprint]. 2021 Apr 12:2021.04.09.439169. doi: 10.1101/2021.04.09.439169. bioRxiv. 2021. Update in: mBio. 2021 Aug 31;12(4):e0209421. doi: 10.1128/mBio.02094-21. PMID: 33851157 Free PMC article. Updated. Preprint.
-
Regulation of Retroviral and SARS-CoV-2 Protease Dimerization and Activity through Reversible Oxidation.Antioxidants (Basel). 2022 Oct 18;11(10):2054. doi: 10.3390/antiox11102054. Antioxidants (Basel). 2022. PMID: 36290777 Free PMC article. Review.
References
-
- Kaplan A H, Manchester M, Everitt L, Swanstrom R. Methods Enzymol. 1994;241:58–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

