A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism
- PMID: 11724939
- PMCID: PMC64730
- DOI: 10.1073/pnas.251465298
A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism
Abstract
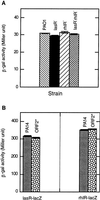
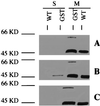
The human opportunistic pathogen Pseudomonas aeruginosa strain PA14 infects both plants and animals. Previously, using plants to screen directly for P. aeruginosa virulence-attenuated mutants, we identified a locus, pho34B12, relevant in mammalian pathogenesis. Here, nonsense point mutations in the two opposing ORFs identified in the pho34B12 locus revealed that one of them, mvfR (multiple virulence factor Regulator), is able to control all of the phenotypes that mutant phoA34B12 displays. Both genetic and biochemical evidence demonstrate that the mvfR gene encodes a LysR-like transcriptional factor that positively regulates the production of elastase, phospholipase, and of the autoinducers, 3oxo-dodecanoyl homoserine lactone (PAI I) and 2-heptyl-3-hydroxy-4-quinolone (PQS), as well as the expression of the phnAB operon, involved in phenazine biosynthesis. We demonstrate that the MvfR protein is membrane-associated and acts as a transcriptional activator until cells reach stationary phase, when a unique negative feedback mechanism is activated to signal the down-regulation of the MvfR protein. This work reveals an unprecedented virulence mechanism of P. aeruginosa and identifies a unique indispensable player in the P. aeruginosa quorum-sensing cascade.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

