Binding specificity of Escherichia coli trigger factor
- PMID: 11724963
- PMCID: PMC64667
- DOI: 10.1073/pnas.261432298
Binding specificity of Escherichia coli trigger factor
Abstract
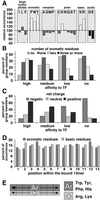
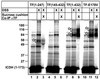
The ribosome-associated chaperone trigger factor (TF) assists the folding of newly synthesized cytosolic proteins in Escherichia coli. Here, we determined the substrate specificity of TF by examining its binding to 2842 membrane-coupled 13meric peptides. The binding motif of TF was identified as a stretch of eight amino acids, enriched in basic and aromatic residues and with a positive net charge. Fluorescence spectroscopy verified that TF exhibited a comparable substrate specificity for peptides in solution. The affinity to peptides in solution was low, indicating that TF requires ribosome association to create high local concentrations of nascent polypeptide substrates for productive interaction in vivo. Binding to membrane-coupled peptides occurred through the central peptidyl-prolyl-cis/trans isomerase (PPIase) domain of TF, however, independently of prolyl residues. Crosslinking experiments showed that a TF fragment containing the PPIase domain linked to the ribosome via the N-terminal domain is sufficient for interaction with nascent polypeptide substrates. Homology modeling of the PPIase domain revealed a conserved FKBP(FK506-binding protein)-like binding pocket composed of exposed aromatic residues embedded in a groove with negative surface charge. The features of this groove complement well the determined substrate specificity of TF. Moreover, a mutation (E178V) in this putative substrate binding groove known to enhance PPIase activity also enhanced TF's association with a prolyl-free model peptide in solution and with nascent polypeptides. This result suggests that both prolyl-independent binding of peptide substrates and peptidyl-prolyl isomerization involve the same binding site.
Figures


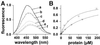
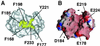
Similar articles
-
Functional dissection of Escherichia coli trigger factor: unraveling the function of individual domains.J Bacteriol. 2004 Jun;186(12):3777-84. doi: 10.1128/JB.186.12.3777-3784.2004. J Bacteriol. 2004. PMID: 15175291 Free PMC article.
-
PPIase domain of trigger factor acts as auxiliary chaperone site to assist the folding of protein substrates bound to the crevice of trigger factor.Int J Biochem Cell Biol. 2010 Jun;42(6):890-901. doi: 10.1016/j.biocel.2010.01.019. Epub 2010 Jan 21. Int J Biochem Cell Biol. 2010. PMID: 20096367
-
Three-state equilibrium of Escherichia coli trigger factor.Biol Chem. 2002 Oct;383(10):1611-9. doi: 10.1515/BC.2002.182. Biol Chem. 2002. PMID: 12452438
-
A cradle for new proteins: trigger factor at the ribosome.Curr Opin Struct Biol. 2005 Apr;15(2):204-12. doi: 10.1016/j.sbi.2005.03.005. Curr Opin Struct Biol. 2005. PMID: 15837180 Review.
-
Peptidyl-prolyl cis-trans isomerases: structure and functions.Biochemistry (Mosc). 1999 Jul;64(7):738-51. Biochemistry (Mosc). 1999. PMID: 10424896 Review.
Cited by
-
Trigger Factor can antagonize both SecB and DnaK/DnaJ chaperone functions in Escherichia coli.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3101-6. doi: 10.1073/pnas.0608232104. Epub 2007 Feb 20. Proc Natl Acad Sci U S A. 2007. PMID: 17360615 Free PMC article.
-
Trigger factor finds new jobs and contacts.Nat Struct Mol Biol. 2009 Oct;16(10):1006-8. doi: 10.1038/nsmb1009-1006. Nat Struct Mol Biol. 2009. PMID: 19809489 No abstract available.
-
In vivo analysis of the overlapping functions of DnaK and trigger factor.EMBO Rep. 2004 Feb;5(2):195-200. doi: 10.1038/sj.embor.7400067. Epub 2004 Jan 9. EMBO Rep. 2004. PMID: 14726952 Free PMC article.
-
Structural characterization of the interaction of α-synuclein nascent chains with the ribosomal surface and trigger factor.Proc Natl Acad Sci U S A. 2016 May 3;113(18):5012-7. doi: 10.1073/pnas.1519124113. Epub 2016 Apr 18. Proc Natl Acad Sci U S A. 2016. PMID: 27092002 Free PMC article.
-
Molecular chaperones facilitate the soluble expression of N-acyl-D-amino acid amidohydrolases in Escherichia coli.J Ind Microbiol Biotechnol. 2004 Oct;31(9):421-6. doi: 10.1007/s10295-004-0163-4. Epub 2004 Aug 28. J Ind Microbiol Biotechnol. 2004. PMID: 15338421
References
-
- Bukau B, Deuerling E, Pfund C, Craig E A. Cell. 2000;101:119–122. - PubMed
-
- Ellis R J, Hemmingsen S M. Trends Biochem Sci. 1989;14:339–342. - PubMed
-
- Gething M-J H, Sambrook J F. Nature (London) 1992;355:33–45. - PubMed
-
- Hartl F U. Nature (London) 1996;381:571–580. - PubMed
-
- Horwich A L, Brooks Low K, Fenton W A, Hirshfield I N, Furtak K. Cell. 1993;74:909–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

