Ceramide-mediated apoptosis in lung epithelial cells is regulated by glutathione
- PMID: 11726392
- PMCID: PMC4370277
- DOI: 10.1165/ajrcmb.25.6.4321
Ceramide-mediated apoptosis in lung epithelial cells is regulated by glutathione
Abstract
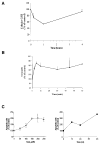
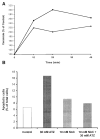
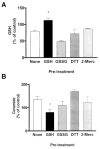
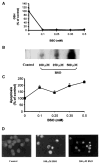
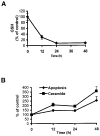
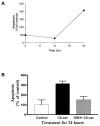
Reactive oxygen species (ROS) are mediators of lung injury, and glutathione (GSH) is the major nonprotein antioxidant that protects the cell from oxidative stress. We have recently shown that H(2)O(2) induces ceramide-mediated apoptosis in human lung epithelial cells. We hypothesized that ROS-mediated depletion of GSH plays a regulatory role in ceramide generation, and thus in the induction of apoptosis. Our present studies demonstrate that GSH at physiologic concentrations (1 to 10 mM) inhibits ceramide production in a time- and dose-dependent manner in A549 human alveolar epithelial cells. On the other hand, buthionine-sulfoximine-mediated depletion of intracellular GSH induces elevation of ceramide levels and apoptosis. In addition, GSH blocks H(2)O(2)-mediated induction of intracellular ceramide generation and apoptosis. These effects were not mimicked by oxidized GSH (GSSG) or other thiol antioxidants, such as dithiothreitol and 2-mercaptoethanol. Moreover, increase of intracellular H(2)O(2), mediated by inhibition of catalase by aminotriazole, also induces ceramide generation and apoptosis. These effects were blocked by N-acetylcysteine. Our results suggest that GSH depletion may be the link between oxidative stress and ceramide-mediated apoptosis in the lung.
Figures



Comment in
-
Reactive oxygen species and cell signaling.Am J Respir Cell Mol Biol. 2001 Dec;25(6):661-3. doi: 10.1165/ajrcmb.25.6.f213. Am J Respir Cell Mol Biol. 2001. PMID: 11726388 No abstract available.
References
-
- Rahman I, Li XY, Donaldson K, Harrison DJ, MacNee W. Glutathione homeostasis in alveolar epithelial cells in vitro and lung in vivo under oxidative stress. Am J Physiol. 1995;269(3 Pt 1):L285–L292. - PubMed
-
- Mulier B, Rahman I, Watchorn T, Donaldson K, MacNee W, Jeffery PK. Hydrogen peroxide-induced epithelial injury: the protective role of intracellular nonprotein thiols (NPSH) Eur Respir J. 1998;11:384–391. - PubMed
-
- Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16:534–554. - PubMed
-
- Goldkorn T, Balaban N, Shannon M, Chea V, Matsukuma K, Gilchrist D, Wang H, Chan C. H2O2 acts on cellular membranes to generate ceramide signaling and initiate apoptosis in tracheobronchial epithelial cells. J Cell Sci. 1998;111(Pt. 21):3209–3220. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

