Nuclear export of phosphorylated C/EBPbeta mediates the inhibition of albumin expression by TNF-alpha
- PMID: 11726507
- PMCID: PMC125761
- DOI: 10.1093/emboj/20.23.6712
Nuclear export of phosphorylated C/EBPbeta mediates the inhibition of albumin expression by TNF-alpha
Abstract
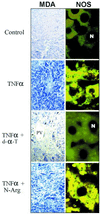
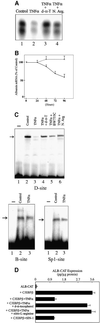
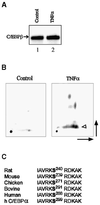
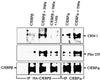
Decreased albumin expression is a frequent feature of cachexia patients afflicted with chronic diseases, including cancer, and a major contributor to their morbidity. Here we show that tumor necrosis-alpha (TNF-alpha) treatment of primary mouse hepatocytes or TNF-alpha overexpression in a mouse model of cachexia induces oxidative stress, nitric oxide synthase (NOS) expression and phosphorylation of C/EBPbeta on Ser239, within the nuclear localization signal, thus inducing its nuclear export, which inhibits transcription from the albumin gene. SIN-1, a NO donor, duplicated the TNF-alpha effects on hepatocytes. We found similar molecular abnormalities in the liver of patients with cancer-cachexia. The cytoplasmic localization and association of C/EBPbeta-PSer239 with CRM1 (exportin-1) in TNF-alpha-treated hepatocytes was inhibited by leptomycin B, a blocker of CRM1 activity. Hepatic cells expressing the non-phosphorylatable C/EBPbeta alanine mutant were refractory to the inhibitory effects of TNF-alpha on albumin transcription since the mutant remained localized to the nucleus. Treatment of TNF-alpha mice with antioxidants or NOS inhibitors prevented phosphorylation of C/EBPbeta on Ser239 and its nuclear export, and rescued the abnormal albumin gene expression.
Figures


Similar articles
-
Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants.EMBO J. 1996 Apr 15;15(8):1753-65. EMBO J. 1996. PMID: 8617220 Free PMC article.
-
Tumor necrosis factor-alpha inhibits albumin gene expression in a murine model of cachexia.J Clin Invest. 1990 Jan;85(1):248-55. doi: 10.1172/JCI114419. J Clin Invest. 1990. PMID: 2295699 Free PMC article.
-
Osteoprotegerin ligand induces beta-casein gene expression through the transcription factor CCAAT/enhancer-binding protein beta.J Biol Chem. 2002 Feb 15;277(7):5339-44. doi: 10.1074/jbc.M108342200. Epub 2001 Nov 28. J Biol Chem. 2002. PMID: 11726661
-
Inhibition of albumin synthesis in chronic diseases: molecular mechanisms.J Clin Gastroenterol. 2005 Apr;39(4 Suppl 2):S143-6. doi: 10.1097/01.mcg.0000155514.17715.39. J Clin Gastroenterol. 2005. PMID: 15758650 Review.
-
[The cell nuclear damage probably induces chronic refractory diseases].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023 Aug 20;41(8):636-640. doi: 10.3760/cma.j.cn121094-20230302-00059. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023. PMID: 37667165 Review. Chinese.
Cited by
-
Decreased Jun-D and myogenin expression in muscle wasting of human cachexia.Am J Physiol Endocrinol Metab. 2009 Aug;297(2):E392-401. doi: 10.1152/ajpendo.90529.2008. Epub 2009 May 26. Am J Physiol Endocrinol Metab. 2009. PMID: 19470832 Free PMC article.
-
Modulation of JC virus transcription by C/EBPbeta.Virus Res. 2009 Dec;146(1-2):97-106. doi: 10.1016/j.virusres.2009.09.005. Epub 2009 Sep 9. Virus Res. 2009. PMID: 19747512 Free PMC article.
-
The Janus-Faced Role of Antioxidants in Cancer Cachexia: New Insights on the Established Concepts.Oxid Med Cell Longev. 2016;2016:9579868. doi: 10.1155/2016/9579868. Epub 2016 Aug 24. Oxid Med Cell Longev. 2016. PMID: 27642498 Free PMC article. Review.
-
Effects of Albumin Infusion on Serum Levels of Albumin, Proinflammatory Cytokines (TNF-α, IL-1, and IL-6), CRP, and MMP-8; Tissue Expression of EGRF, ERK1, ERK2, TGF-β, Collagen, and MMP-8; and Wound Healing in Sprague Dawley Rats.Int J Inflam. 2020 May 20;2020:3254017. doi: 10.1155/2020/3254017. eCollection 2020. Int J Inflam. 2020. PMID: 32518615 Free PMC article.
-
Severe hepatocellular injury with apoptosis induced by a hepatitis C polymerase inhibitor.J Clin Gastroenterol. 2009 Apr;43(4):374-81. doi: 10.1097/MCG.0b013e318178d91f. J Clin Gastroenterol. 2009. PMID: 19098685 Free PMC article.
References
-
- Akira S., Hirano,T., Taga,T. and Kishimoto,T. (1990b) Biology of multifunctional cytokines; IL 6 and related molecules (IL and TNF). FASEB J., 4, 2860–2867. - PubMed
-
- Beutler B. (1992) Tumor Necrosis Factors: The Molecules and Their Emerging Role in Medicine. Raven Press, New York.
-
- Boyle W.J., van der Geer,P. and Hunter,T. (1991) Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol., 201, 110–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

