An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export
- PMID: 11726510
- PMCID: PMC125768
- DOI: 10.1093/emboj/20.23.6742
An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export
Abstract
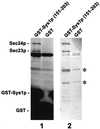
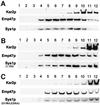
We previously identified Sys1p as a high copy number suppressor of Ypt6 GTPase-deficient yeast mutants that are defective in endosome-to-Golgi transport. Here, we show that Sys1p is an integral membrane protein that resides on a post-endoplasmic reticulum (ER) organelle(s). Affinity studies with detergent- solubilized yeast proteins showed that the C-terminal 53 amino acid tail of Sys1p binds effectively to the cytoplasmic Sec23p-Sec24p COPII subcomplex. This binding required a di-acidic Asp-Leu-Glu (DXE) motif, previously shown to mediate efficient ER export of the vesicular stomatitis virus glycoprotein in mammalian cells. In Sys1p, a Glu-Leu-Glu (EXE) sequence could not substitute for the (DXE) motif. Mutations of the (DXE) sequence resulted in ER retention of approximately 30% of the protein at steady state, whereas addition of the Sys1p tail to an ER-resident membrane protein led to an intracellular redistribution of the chimeric protein. Our study demonstrates for the first time that, in yeast, a di-acidic sequence motif can act as a sorting signal for cargo selection during the formation of transport vesicles at the ER by direct binding to COPII component(s).
Figures






Similar articles
-
Mammalian Bet3 functions as a cytosolic factor participating in transport from the ER to the Golgi apparatus.J Cell Sci. 2005 Mar 15;118(Pt 6):1209-22. doi: 10.1242/jcs.01723. Epub 2005 Feb 22. J Cell Sci. 2005. PMID: 15728249
-
The GTPase Arf1p and the ER to Golgi cargo receptor Erv14p cooperate to recruit the golgin Rud3p to the cis-Golgi.J Cell Biol. 2004 Oct 25;167(2):281-92. doi: 10.1083/jcb.200407088. J Cell Biol. 2004. PMID: 15504911 Free PMC article.
-
Sterols regulate ER-export dynamics of secretory cargo protein ts-O45-G.EMBO J. 2006 Jul 12;25(13):2953-65. doi: 10.1038/sj.emboj.7601205. Epub 2006 Jun 22. EMBO J. 2006. PMID: 16794576 Free PMC article.
-
Mechanisms of COPII vesicle formation and protein sorting.FEBS Lett. 2007 May 22;581(11):2076-82. doi: 10.1016/j.febslet.2007.01.091. Epub 2007 Feb 14. FEBS Lett. 2007. PMID: 17316621 Review.
-
A structural view of the COPII vesicle coat.Curr Opin Struct Biol. 2004 Apr;14(2):147-53. doi: 10.1016/j.sbi.2004.02.002. Curr Opin Struct Biol. 2004. PMID: 15093828 Review.
Cited by
-
Svp26 facilitates endoplasmic reticulum to golgi transport of a set of mannosyltransferases in Saccharomyces cerevisiae.J Biol Chem. 2010 May 14;285(20):15420-15429. doi: 10.1074/jbc.M109.086272. Epub 2010 Mar 17. J Biol Chem. 2010. PMID: 20236934 Free PMC article.
-
The B7-1 cytoplasmic tail enhances intracellular transport and mammalian cell surface display of chimeric proteins in the absence of a linear ER export motif.PLoS One. 2013 Sep 20;8(9):e75084. doi: 10.1371/journal.pone.0075084. eCollection 2013. PLoS One. 2013. PMID: 24073236 Free PMC article.
-
Functional genomics of monensin sensitivity in yeast: implications for post-Golgi traffic and vacuolar H+-ATPase function.Mol Genet Genomics. 2008 Sep;280(3):233-48. doi: 10.1007/s00438-008-0359-9. Epub 2008 Jul 9. Mol Genet Genomics. 2008. PMID: 18612650
-
Di-acidic motifs in the membrane-distal C termini modulate the transport of angiotensin II receptors from the endoplasmic reticulum to the cell surface.J Biol Chem. 2011 Jun 10;286(23):20525-35. doi: 10.1074/jbc.M111.222034. Epub 2011 Apr 20. J Biol Chem. 2011. PMID: 21507945 Free PMC article.
-
A novel di-acidic motif facilitates ER export of the syntaxin SYP31.J Exp Bot. 2009;60(11):3157-65. doi: 10.1093/jxb/erp155. Epub 2009 Jun 10. J Exp Bot. 2009. PMID: 19516076 Free PMC article.
References
-
- Aridor M., Bannykh,S.I., Rowe,T. and Balch,W.E. (1999) Cargo can modulate COPII vesicle formation from the endoplasmic reticulum. J. Biol. Chem., 274, 4389–4399. - PubMed
-
- Balch W.E., McCaffery,J.M., Plutner,H. and Farquhar,M.G. (1994) Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell, 76, 841–852. - PubMed
-
- Ballensiefen W., Ossipov,D. and Schmitt,H.D. (1998) Recycling of the yeast v-SNARE Sec22p involves COPI-proteins and the ER transmembrane proteins Ufe1p and Sec20p. J. Cell Sci., 111, 1507–1520. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

