Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis
- PMID: 11726513
- PMCID: PMC125758
- DOI: 10.1093/emboj/20.23.6772
Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis
Abstract
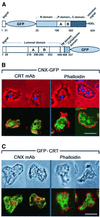
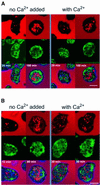
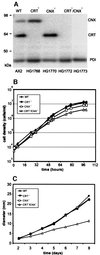
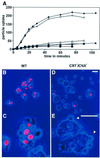
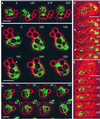
Calreticulin and calnexin are Ca2+-binding proteins with chaperone activity in the endoplasmic reticulum. These proteins have been eliminated by gene replacement in Dictyostelium, the only microorganism known to harbor both proteins; family members in Dictyostelium are located at the base of phylogenetic trees. A dramatic decline in the rate of phagocytosis was observed in double mutants lacking calreticulin and calnexin, whereas only mild changes occurred in single mutants. Dictyostelium cells are professional phagocytes, capable of internalizing particles by a sequence of activities: adhesion of the particle to the cell surface, actin-dependent outgrowth of a phagocytic cup, and separation of the phagosome from the plasma membrane. In the double-null mutants, particles still adhered to the cell surface, but the outgrowth of phagocytic cups was compromised. Green fluorescent protein-tagged calreticulin and calnexin, expressed in wild-type cells, revealed a direct link of the endoplasmic reticulum to the phagocytic cup enclosing a particle, such that the Ca2+ storage capacity of calreticulin and calnexin might directly modulate activities of the actin system during particle uptake.
Figures


References
-
- Aizawa H., Sutoh,K., Tsubuki,S., Kawashima,S., Ishii,A. and Yahara,I. (1995) Identification, characterization and intracellular distribution of cofilin in Dictyostelium discoideum. J. Biol. Chem., 270, 10923–10932. - PubMed
-
- Arima H., Kinoshita,T., Ibrahim,H.R., Azakami,H. and Kato,A. (1998) Enhanced secretion of hydrophobic peptide fused lysozyme by the introduction of N-glycosylation signal and the disruption of calnexin gene in Saccharomyces cerevisiae. FEBS Lett., 440, 89–92. - PubMed
-
- Aubry L., Klein,G., Martiel,J.-L. and Satre,M. (1997) Fluid-phase endocytosis in the amoebae of the cellular slime mould Dictyostelium discoideum: mathematical modelling of kinetics and pH evolution. J. Theor. Biol., 184, 89–98.
-
- Cardelli J. (2001) Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic, 2, 311–320. - PubMed
-
- Coppolino M.G., Woodside,M.J., Demaurex,N., Grinstein,S., St-Arnaud,R. and Dedhar,S. (1997) Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature, 386, 843–847. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

