Tyrosine phosphorylation of Grb2 by Bcr/Abl and epidermal growth factor receptor: a novel regulatory mechanism for tyrosine kinase signaling
- PMID: 11726515
- PMCID: PMC125747
- DOI: 10.1093/emboj/20.23.6793
Tyrosine phosphorylation of Grb2 by Bcr/Abl and epidermal growth factor receptor: a novel regulatory mechanism for tyrosine kinase signaling
Abstract
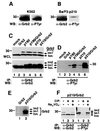
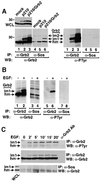
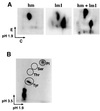
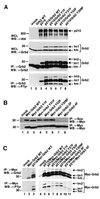
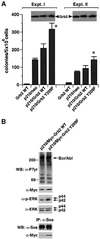
Growth factor receptor-binding protein-2 (Grb2) plays a key role in signal transduction initiated by Bcr/Abl oncoproteins and growth factors, functioning as an adaptor protein through its Src homology 2 and 3 (SH2 and SH3) domains. We found that Grb2 was tyrosine-phosphorylated in cells expressing BCR/ABL and in A431 cells stimulated with epidermal growth factor (EGF). Phosphorylation of Grb2 by Bcr/Abl or EGF receptor reduced its SH3-dependent binding to Sos in vivo, but not its SH2-dependent binding to Bcr/Abl. Tyr209 within the C-terminal SH3 domain of Grb2 was identified as one of the tyrosine phosphorylation sites, and phosphorylation of Tyr209 abolished the binding of the SH3 domain to a proline-rich Sos peptide in vitro. In vivo expression of a Grb2 mutant where Tyr209 was changed to phenylalanine enhanced BCR/ABL-induced ERK activation and fibroblast transformation, and potentiated and prolonged Grb2-mediated activation of Ras, mitogen-activated protein kinase and c-Jun N-terminal kinase in response to EGF stimulation. These results suggest that tyrosine phosphorylation of Grb2 is a novel mechanism of down-regulation of tyrosine kinase signaling.
Figures



References
-
- Baltensperger K., Kozma,L.M., Cherniack,A.D., Klarlund,J., Chawla,A., Banerjee,U. and Czech,M.P. (1993) Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science, 260, 1950–1952. - PubMed
-
- Benjamin C.W., Linseman,D.A. and Jones,D.A. (1994) Platelet-derived growth factor stimulates phosphorylation of growth factor receptor-binding protein-2 in vascular smooth muscle cells. J. Biol. Chem., 269, 31346–31349. - PubMed
-
- Boyle W.J., van Der Greer,P. and Hunter,T. (1991) Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol., 201, 110–149. - PubMed
-
- Brasher B.B. and Van Etten,R.A. (2000) c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem., 275, 35631–35637. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

