Misreading of termination codons in eukaryotes by natural nonsense suppressor tRNAs
- PMID: 11726686
- PMCID: PMC96686
- DOI: 10.1093/nar/29.23.4767
Misreading of termination codons in eukaryotes by natural nonsense suppressor tRNAs
Abstract
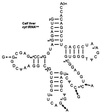
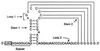
Translational stop codon readthrough provides a regulatory mechanism of gene expression that is extensively utilised by positive-sense ssRNA viruses. The misreading of termination codons is achieved by a variety of naturally occurring suppressor tRNAs whose structure and function is the subject of this survey. All of the nonsense suppressors characterised to date (with the exception of selenocysteine tRNA) are normal cellular tRNAs that are primarily needed for reading their cognate sense codons. As a consequence, recognition of stop codons by natural suppressor tRNAs necessitates unconventional base pairings in anticodon-codon interactions. A number of intrinsic features of the suppressor tRNA contributes to the ability to read non-cognate codons. Apart from anticodon-codon affinity, the extent of base modifications within or 3' of the anticodon may up- or down-regulate the efficiency of suppression. In order to out-compete the polypeptide chain release factor an absolute prerequisite for the action of natural suppressor tRNAs is a suitable nucleotide context, preferentially at the 3' side of the suppressed stop codon. Three major types of viral readthrough sites, based on similar sequences neighbouring the leaky stop codon, can be defined. It is discussed that not only RNA viruses, but also the eukaryotic host organism might gain some profit from cellular suppressor tRNAs.
Figures







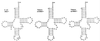


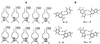



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

