Molecular recognition of pyr mRNA by the Bacillus subtilis attenuation regulatory protein PyrR
- PMID: 11726695
- PMCID: PMC96680
- DOI: 10.1093/nar/29.23.4851
Molecular recognition of pyr mRNA by the Bacillus subtilis attenuation regulatory protein PyrR
Abstract
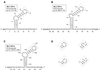
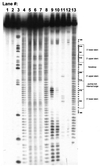
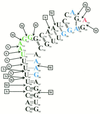
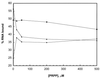
The pyrimidine nucleotide biosynthesis (pyr) operon in Bacillus subtilis is regulated by transcriptional attenuation. The PyrR protein binds in a uridine nucleotide-dependent manner to three attenuation sites at the 5'-end of pyr mRNA. PyrR binds an RNA-binding loop, allowing a terminator hairpin to form and repressing the downstream genes. The binding of PyrR to defined RNA molecules was characterized by a gel mobility shift assay. Titration indicated that PyrR binds RNA in an equimolar ratio. PyrR bound more tightly to the binding loops from the second (BL2 RNA) and third (BL3 RNA) attenuation sites than to the binding loop from the first (BL1 RNA) attenuation site. PyrR bound BL2 RNA 4-5-fold tighter in the presence of saturating UMP or UDP and 150- fold tighter with saturating UTP, suggesting that UTP is the more important co-regulator. The minimal RNA that bound tightly to PyrR was 28 nt long. Thirty-one structural variants of BL2 RNA were tested for PyrR binding affinity. Two highly conserved regions of the RNA, the terminal loop and top of the upper stem and a purine-rich internal bulge and the base pairs below it, were crucial for tight binding. Conserved elements of RNA secondary structure were also required for tight binding. PyrR protected conserved areas of the binding loop in hydroxyl radical footprinting experiments. PyrR likely recognizes conserved RNA sequences, but only if they are properly positioned in the correct secondary structure.
Figures







References
-
- Quinn C.L., Stephenson,B.T. and Switzer,R.L. (1991) Functional organization and nucleotide sequence of the Bacillus subtilis pyrimidine biosynthetic operon. J. Biol. Chem., 266, 9113–9127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

