Biodegradation of aromatic compounds by Escherichia coli
- PMID: 11729263
- PMCID: PMC99040
- DOI: 10.1128/MMBR.65.4.523-569.2001
Biodegradation of aromatic compounds by Escherichia coli
Abstract
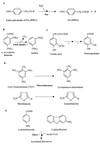
Although Escherichia coli has long been recognized as the best-understood living organism, little was known about its abilities to use aromatic compounds as sole carbon and energy sources. This review gives an extensive overview of the current knowledge of the catabolism of aromatic compounds by E. coli. After giving a general overview of the aromatic compounds that E. coli strains encounter and mineralize in the different habitats that they colonize, we provide an up-to-date status report on the genes and proteins involved in the catabolism of such compounds, namely, several aromatic acids (phenylacetic acid, 3- and 4-hydroxyphenylacetic acid, phenylpropionic acid, 3-hydroxyphenylpropionic acid, and 3-hydroxycinnamic acid) and amines (phenylethylamine, tyramine, and dopamine). Other enzymatic activities acting on aromatic compounds in E. coli are also reviewed and evaluated. The review also reflects the present impact of genomic research and how the analysis of the whole E. coli genome reveals novel aromatic catabolic functions. Moreover, evolutionary considerations derived from sequence comparisons between the aromatic catabolic clusters of E. coli and homologous clusters from an increasing number of bacteria are also discussed. The recent progress in the understanding of the fundamentals that govern the degradation of aromatic compounds in E. coli makes this bacterium a very useful model system to decipher biochemical, genetic, evolutionary, and ecological aspects of the catabolism of such compounds. In the last part of the review, we discuss strategies and concepts to metabolically engineer E. coli to suit specific needs for biodegradation and biotransformation of aromatics and we provide several examples based on selected studies. Finally, conclusions derived from this review may serve as a lead for future research and applications.
Figures








References
-
- Alekshun M N, Kim Y S, Levy S B. Mutational analysis of MarR, the negative regulator of marRAB expression in Escherichia coli, suggests the presence of two regions required for DNA binding. Mol Microbiol. 2000;35:1394–1404. - PubMed
-
- Allende J L, Gibello A, Martin M, Garrido-Pertierra A. Transport of 4-hydroxyphenylacetic acid in Klebsiella pneumoniae. Arch Biochem Biophys. 1992;292:583–588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

