Transport into and out of the nucleus
- PMID: 11729264
- PMCID: PMC99041
- DOI: 10.1128/MMBR.65.4.570-594.2001
Transport into and out of the nucleus
Abstract
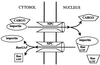
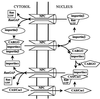
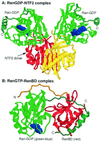
A defining characteristic of eukaryotic cells is the possession of a nuclear envelope. Transport of macromolecules between the nuclear and cytoplasmic compartments occurs through nuclear pore complexes that span the double membrane of this envelope. The molecular basis for transport has been revealed only within the last few years. The transport mechanism lacks motors and pumps and instead operates by a process of facilitated diffusion of soluble carrier proteins, in which vectoriality is provided by compartment-specific assembly and disassembly of cargo-carrier complexes. The carriers recognize localization signals on the cargo and can bind to pore proteins. They also bind a small GTPase, Ran, whose GTP-bound form is predominantly nuclear. Ran-GTP dissociates import carriers from their cargo and promotes the assembly of export carriers with cargo. The ongoing discovery of numerous carriers, Ran-independent transport mechanisms, and cofactors highlights the complexity of the nuclear transport process. Multiple regulatory mechanisms are also being identified that control cargo-carrier interactions. Circadian rhythms, cell cycle, transcription, RNA processing, and signal transduction are all regulated at the level of nucleocytoplasmic transport. This review focuses on recent discoveries in the field, with an emphasis on the carriers and cofactors involved in transport and on possible mechanisms for movement through the nuclear pores.
Figures







References
-
- Adam S A, Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–847. - PubMed
-
- Ahmadian M R, Stege P, Scheffzek K, Wittinghofer A. Confirmation of the arginine-finger hypothesis for the GAP-stimulated GTP-hydrolysis reaction of Ras. Nat Struct Biol. 1997;4:686–689. - PubMed
-
- Aitchison J D, Blobel G, Rout M P. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

