Actin-based motility of intracellular microbial pathogens
- PMID: 11729265
- PMCID: PMC99042
- DOI: 10.1128/MMBR.65.4.595-626.2001
Actin-based motility of intracellular microbial pathogens
Abstract
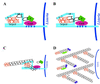
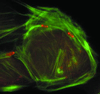
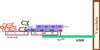
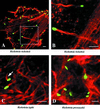
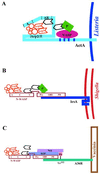
A diverse group of intracellular microorganisms, including Listeria monocytogenes, Shigella spp., Rickettsia spp., and vaccinia virus, utilize actin-based motility to move within and spread between mammalian host cells. These organisms have in common a pathogenic life cycle that involves a stage within the cytoplasm of mammalian host cells. Within the cytoplasm of host cells, these organisms activate components of the cellular actin assembly machinery to induce the formation of actin tails on the microbial surface. The assembly of these actin tails provides force that propels the organisms through the cell cytoplasm to the cell periphery or into adjacent cells. Each of these organisms utilizes preexisting mammalian pathways of actin rearrangement to induce its own actin-based motility. Particularly remarkable is that while all of these microbes use the same or overlapping pathways, each intercepts the pathway at a different step. In addition, the microbial molecules involved are each distinctly different from the others. Taken together, these observations suggest that each of these microbes separately and convergently evolved a mechanism to utilize the cellular actin assembly machinery. The current understanding of the molecular mechanisms of microbial actin-based motility is the subject of this review.
Figures




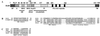



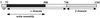





Similar articles
-
Molecular mechanisms of cell-cell spread of intracellular bacterial pathogens.Open Biol. 2013 Jul 17;3(7):130079. doi: 10.1098/rsob.130079. Open Biol. 2013. PMID: 23864553 Free PMC article. Review.
-
Tyrosine phosphorylation is required for actin-based motility of vaccinia but not Listeria or Shigella.Curr Biol. 1999 Jan 28;9(2):89-92. doi: 10.1016/s0960-9822(99)80020-7. Curr Biol. 1999. PMID: 10021367
-
Actin cytoskeleton. Missing link for intracellular bacterial motility?Curr Biol. 1995 Aug 1;5(8):837-40. doi: 10.1016/s0960-9822(95)00167-9. Curr Biol. 1995. PMID: 7583135
-
Interaction of vaccinia virus with the actin cytoskeleton.Folia Microbiol (Praha). 1998;43(3):305-10. doi: 10.1007/BF02818616. Folia Microbiol (Praha). 1998. PMID: 9717258 Review.
-
Surfing pathogens and the lessons learned for actin polymerization.Trends Cell Biol. 2001 Jan;11(1):30-38. doi: 10.1016/s0962-8924(00)01871-7. Trends Cell Biol. 2001. PMID: 11146296 Review.
Cited by
-
Interaction between the membrane protein of a pathogen and insect microfilament complex determines insect-vector specificity.Proc Natl Acad Sci U S A. 2006 Mar 14;103(11):4252-7. doi: 10.1073/pnas.0508668103. Epub 2006 Mar 6. Proc Natl Acad Sci U S A. 2006. PMID: 16537517 Free PMC article.
-
Type IV Pili Can Mediate Bacterial Motility within Epithelial Cells.mBio. 2019 Aug 20;10(4):e02880-18. doi: 10.1128/mBio.02880-18. mBio. 2019. PMID: 31431558 Free PMC article.
-
Pseudomonas aeruginosa Can Diversify after Host Cell Invasion to Establish Multiple Intracellular Niches.mBio. 2022 Dec 20;13(6):e0274222. doi: 10.1128/mbio.02742-22. Epub 2022 Nov 14. mBio. 2022. PMID: 36374039 Free PMC article.
-
Outer membrane protein A (OmpA): a new player in shigella flexneri protrusion formation and inter-cellular spreading.PLoS One. 2012;7(11):e49625. doi: 10.1371/journal.pone.0049625. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23166731 Free PMC article.
-
VirB-mediated positive feedback control of the virulence gene regulatory cascade of Shigella flexneri.J Bacteriol. 2012 Oct;194(19):5264-73. doi: 10.1128/JB.00800-12. Epub 2012 Jul 20. J Bacteriol. 2012. PMID: 22821978 Free PMC article.
References
-
- Ahern-Djamali S M, Comer A R, Bachmann C, Kastenmeier A S, Reddy S K, Beckerle M C, Walter U, Hoffmann F M. Mutations in Drosophila Enabled and rescue by human vasodilator-stimulated phosphoprotein (VASP) indicate important functional roles for Ena/VASP homology domain 1 (EVH1) and EVH2 domains. Mol Biol Cell. 1998;9:2157–2171. - PMC - PubMed
-
- Amann K J, Pollard T D. The Arp2/3 complex nucleates actin filament branches from the sides of pre-existing filaments. Nat Cell Biol. 2001;3:306–310. - PubMed
-
- Anton I M, Lu W, Mayer R J, Ramesh N, Geha R S. The Wiskott-Aldrich syndrome protein-interacting protein (WIP) binds to the adaptor protein Nck. J Biol Chem. 1998;273:20992–20995. - PubMed
-
- Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding and actin bundle formation. J Biol Chem. 1999;274:23549–23557. - PubMed
-
- Bailly M, Ichetovkin I, Grant W, Zebda N, Machesky L M, Segall J E, Condeelis J. The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol. 2001;11:620–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

