Mathematical modelling of non-stationary fluctuation analysis for studying channel properties of synaptic AMPA receptors
- PMID: 11731574
- PMCID: PMC2278972
- DOI: 10.1111/j.1469-7793.2001.00407.x
Mathematical modelling of non-stationary fluctuation analysis for studying channel properties of synaptic AMPA receptors
Abstract
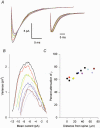
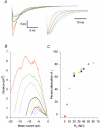
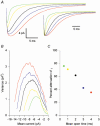
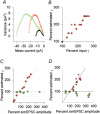
1. The molecular properties of synaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors are an important factor determining excitatory synaptic transmission in the brain. Changes in the number (N) or single-channel conductance (gamma) of functional AMPA receptors may underlie synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD). These parameters have been estimated using non-stationary fluctuation analysis (NSFA). 2. The validity of NSFA for studying the channel properties of synaptic AMPA receptors was assessed using a cable model with dendritic spines and a microscopic kinetic description of AMPA receptors. Electrotonic, geometric and kinetic parameters were altered in order to determine their effects on estimates of the underlying gamma. 3. Estimates of gamma were very sensitive to the access resistance of the recording (R(A)) and the mean open time of AMPA channels. Estimates of gamma were less sensitive to the distance between the electrode and the synaptic site, the electrotonic properties of dendritic structures, recording electrode capacitance and background noise. Estimates of gamma were insensitive to changes in spine morphology, synaptic glutamate concentration and the peak open probability (P(o)) of AMPA receptors. 4. The results obtained using the model agree with biological data, obtained from 91 dendritic recordings from rat CA1 pyramidal cells. A correlation analysis showed that R(A) resulted in a slowing of the decay time constant of excitatory postsynaptic currents (EPSCs) by approximately 150 %, from an estimated value of 3.1 ms. R(A) also greatly attenuated the absolute estimate of gamma by approximately 50-70 %. 5. When other parameters remain constant, the model demonstrates that NSFA of dendritic recordings can readily discriminate between changes in gamma vs. changes in N or P(o). Neither background noise nor asynchronous activation of multiple synapses prevented reliable discrimination between changes in gamma and changes in either N or P(o). 6. The model (available online) can be used to predict how changes in the different properties of AMPA receptors may influence synaptic transmission and plasticity.
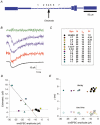
Figures
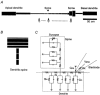
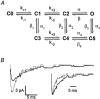
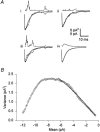

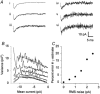

References
-
- Benke TA, Lüthi A, Isaac JTR, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;395:793–797. - PubMed
-
- Bliss T, Collingridge G. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Burden RL, Faires JD. Numerical Analysis. PWS, Boston: 1985. Interpolation and polynomial approximation; pp. 78–135.
-
- Choi S, Klingauf J, Tsien RW. Postfusional regulation of cleft glutamate concentration during LTP at ‘silent synapses’. Nature Neuroscience. 2000;3:330–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

