Effects of paramyxoviral infection on airway epithelial cell Foxj1 expression, ciliogenesis, and mucociliary function
- PMID: 11733356
- PMCID: PMC1850590
- DOI: 10.1016/S0002-9440(10)63057-X
Effects of paramyxoviral infection on airway epithelial cell Foxj1 expression, ciliogenesis, and mucociliary function
Abstract
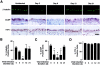
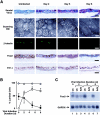
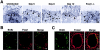
To elucidate molecular mechanisms underlying the association between respiratory viral infection and predisposition to subsequent bacterial infection, we used in vivo and in vitro models and human samples to characterize respiratory virus-induced changes in airway epithelial cell morphology, gene expression, and mucociliary function. Mouse paramyxoviral bronchitis resulted in airway epithelial cell infection and a distinct pattern of epithelial cell morphology changes and altered expression of the differentiation markers beta-tubulin-IV, Clara cell secretory protein, and Foxj1. Furthermore, changes in gene expression were recapitulated using an in vitro epithelial cell culture system and progressed independent of the host inflammatory response. Restoration of mature airway epithelium occurred in a pattern similar to epithelial cell differentiation and ciliogenesis in embryonic lung development characterized by sequential proliferation of undifferentiated cells, basal body production, Foxj1 expression, and beta-tubulin-IV expression. The effects of virus-induced alterations in morphology and gene expression on epithelial cell function were illustrated by decreased airway mucociliary velocity and impaired bacterial clearance. Similar changes in epithelial cell Foxj1 expression were also observed in human paramyxoviral respiratory infection. Taken together, these model systems of paramyxoviral respiratory infection mimic human pathology and identify epithelial cell Foxj1 expression as an early marker of epithelial cell differentiation, recovery, and function.
Figures


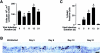

Similar articles
-
Foxj1 regulates basal body anchoring to the cytoskeleton of ciliated pulmonary epithelial cells.J Cell Sci. 2004 Mar 15;117(Pt 8):1329-37. doi: 10.1242/jcs.00978. Epub 2004 Mar 2. J Cell Sci. 2004. PMID: 14996907
-
Foxj1 is required for apical localization of ezrin in airway epithelial cells.J Cell Sci. 2003 Dec 15;116(Pt 24):4935-45. doi: 10.1242/jcs.00830. J Cell Sci. 2003. PMID: 14625387
-
Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2004 Apr;286(4):L650-7. doi: 10.1152/ajplung.00170.2003. Epub 2003 Jun 20. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 12818891
-
Tumor necrosis factor alpha stimulation of human Clara cell secretory protein production by human airway epithelial cells.Ann N Y Acad Sci. 2000;923:193-201. doi: 10.1111/j.1749-6632.2000.tb05530.x. Ann N Y Acad Sci. 2000. PMID: 11193757 Review.
-
Leveraging 3D Model Systems to Understand Viral Interactions with the Respiratory Mucosa.Viruses. 2020 Dec 11;12(12):1425. doi: 10.3390/v12121425. Viruses. 2020. PMID: 33322395 Free PMC article. Review.
Cited by
-
RFX3 modulation of FOXJ1 regulation of cilia genes in the human airway epithelium.Respir Res. 2013 Jul 3;14(1):70. doi: 10.1186/1465-9921-14-70. Respir Res. 2013. PMID: 23822649 Free PMC article.
-
In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19.Mod Pathol. 2020 Nov;33(11):2104-2114. doi: 10.1038/s41379-020-0595-z. Epub 2020 Jun 19. Mod Pathol. 2020. PMID: 32561849 Free PMC article.
-
ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia.J Virol. 2005 Dec;79(23):14614-21. doi: 10.1128/JVI.79.23.14614-14621.2005. J Virol. 2005. PMID: 16282461 Free PMC article.
-
In vitro models to study viral-induced asthma exacerbation: a short review for a key issue.Front Allergy. 2025 Mar 28;6:1530122. doi: 10.3389/falgy.2025.1530122. eCollection 2025. Front Allergy. 2025. PMID: 40224321 Free PMC article. Review.
-
Cilia-related gene signature in the nasal mucosa correlates with disease severity and outcomes in critical respiratory syncytial virus bronchiolitis.Front Immunol. 2022 Sep 23;13:924792. doi: 10.3389/fimmu.2022.924792. eCollection 2022. Front Immunol. 2022. PMID: 36211387 Free PMC article.
References
-
- Zhang P, Summer WR, Bagby GJ, Nelson S: Innate immunity and pulmonary host defense. Immunol Rev 2000, 173:39-51 - PubMed
-
- Frick AG, Joseph TD, Pang L, Rabe AM, St Geme JW, III, Look DC: Haemophilus influenzae stimulates ICAM-1 expression on respiratory epithelial cells. J Immunol 2000, 164:4185-4196 - PubMed
-
- Wanner A, Salathé M, O’Riordan TG: Mucociliary clearance in the airway. Am J Respir Crit Care Med 1996, 154:1868-1902 - PubMed
-
- Erjefalt JS, Erjefalt I, Sundler F, Persson CG: In vivo restitution of airway epithelium. Cell Tissue Res 1995, 281:305-316 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

