Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids
- PMID: 11733357
- PMCID: PMC1850600
- DOI: 10.1016/s0002-9440(10)63058-1
Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids
Abstract
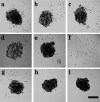
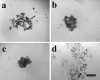
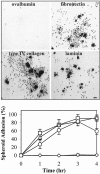
Ovarian carcinoma multicellular spheroids are an in vitro model of micrometastasis whose adhesive abilities have not been elucidated. In this study, we identified adhesion molecules that mediate the formation of ovarian carcinoma spheroids and their subsequent adhesion to extracellular matrix proteins. The NIH:OVCAR5, but not the SKOV3, ovarian carcinoma cell line formed spheroids similar to multicellular aggregates isolated from patient ascitic fluid. NIH:OVCAR5 spheroid formation was augmented by a beta 1-integrin-stimulating monoclonal antibody or exogenous fibronectin, but was inhibited by blocking monoclonal antibodies against the alpha 5- or beta 1-integrin subunits. By immunohistochemical staining, alpha 2-, alpha 3-, alpha 5-, alpha 6-, and beta 1-integrin subunits, CD44, and fibronectin were detected in NIH:OVCAR5 spheroids. NIH:OVCAR5 spheroids adhered to fibronectin, laminin, and type IV collagen, and this adhesion was partially inhibited by blocking antibodies against the alpha 5-, alpha 6-, and alpha 2-integrin subunits, respectively. A blocking monoclonal antibody against the beta 1-integrin subunit completely inhibited adhesion of the spheroids to all three proteins. These results suggest that interactions between the alpha 5 beta 1-integrin and fibronectin mediate the formation of ovarian carcinoma spheroids and that their adhesion to extracellular matrix proteins at sites of secondary tumor growth may be mediated by a complex interaction between multiple integrins and their ligands.
Figures





References
-
- Greenlee RT, Murray T, Bolden S, Wingo PA: Cancer statistics, 2000. CA Cancer J Clin 2000, 50:7-33 - PubMed
-
- Allen HJ, Porter C, Gamarra M, Johnson EA: Isolation and morphologic characterization of human ovarian carcinoma cell clusters present in effusions. Exp Cell Biol 1987, 55:194-208 - PubMed
-
- Sutherland RM: Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 1988, 240:177-184 - PubMed
-
- Filippovich IV, Sorokina NI, Robillard N, Chatal JF: Radiation-induced apoptosis in human ovarian carcinoma cells growing as a monolayer and as multicell spheroids. Int J Cancer 1997, 72:851-859 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

