Rapid response of identified resident endoneurial macrophages to nerve injury
- PMID: 11733369
- PMCID: PMC1850587
- DOI: 10.1016/S0002-9440(10)63070-2
Rapid response of identified resident endoneurial macrophages to nerve injury
Abstract
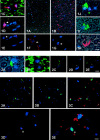
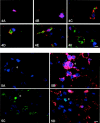
Macrophages play a central role in the pathogenesis of peripheral neuropathy but the role of resident endoneurial macrophages is undefined because no discriminating markers exist to distinguish them from infiltrating hematogenous macrophages. We identified and characterized resident endoneurial macrophages during Wallerian degeneration in radiation bone marrow chimeric rats created by transplanting wild-type Lewis rat bone marrow into irradiated TK-tsa transgenic Lewis rats. In such animals, resident cells carry the transgene, whereas hematogenous cells do not. As early as 2 days after sciatic nerve crush and before the influx of hematogenous macrophages, resident transgene-positive endoneurial macrophages underwent morphological and immunophenotypic signs of activation. At the same time, resident macrophages phagocytosing myelin were found, and proliferation was detected by bromodeoxyuridine incorporation. Continuous bromodeoxyuridine feeding revealed that resident endoneurial macrophages sequentially retracted their processes, proliferated, and expressed the ED1 antigen, rendering them morphologically indistinguishable from hematogenous macrophages. Resident endoneurial macrophages thus play an early and active role in the cellular events after nerve lesion before hematogenous macrophages enter the nerve. They may thus be critically involved in the pathogenesis of peripheral neuropathy particularly at early stages of the disease and may act as sensors of pathology much like their central nervous system counterparts, the microglial cells.
Figures



Similar articles
-
Contribution of resident endoneurial macrophages to the local cellular response in experimental autoimmune neuritis.J Neuropathol Exp Neurol. 2006 May;65(5):499-507. doi: 10.1097/01.jnen.0000229239.43866.d1. J Neuropathol Exp Neurol. 2006. PMID: 16772873
-
Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages.Lab Invest. 2003 Feb;83(2):175-85. doi: 10.1097/01.lab.0000056993.28149.bf. Lab Invest. 2003. PMID: 12594233
-
Lesion response of long-term and recently immigrated resident endoneurial macrophages in peripheral nerve explant cultures from bone marrow chimeric mice.Eur J Neurosci. 2002 Nov;16(9):1654-60. doi: 10.1046/j.1460-9568.2002.02236.x. Eur J Neurosci. 2002. PMID: 12431217
-
Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration.Microsc Res Tech. 2002 Jun 15;57(6):541-7. doi: 10.1002/jemt.10108. Microsc Res Tech. 2002. PMID: 12112437 Review.
-
Myelin and macrophages in the PNS: An intimate relationship in trauma and disease.Brain Res. 2016 Jun 15;1641(Pt A):130-138. doi: 10.1016/j.brainres.2015.11.033. Epub 2015 Nov 26. Brain Res. 2016. PMID: 26631844 Review.
Cited by
-
Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis.J Neuroinflammation. 2010 Jan 28;7:8. doi: 10.1186/1742-2094-7-8. J Neuroinflammation. 2010. PMID: 20109233 Free PMC article.
-
Histological Consequences of Needle-Nerve Contact following Nerve Stimulation in a Pig Model.Anesthesiol Res Pract. 2011;2011:591851. doi: 10.1155/2011/591851. Epub 2011 Apr 19. Anesthesiol Res Pract. 2011. PMID: 21716736 Free PMC article.
-
Nerve cross-bridging to enhance nerve regeneration in a rat model of delayed nerve repair.PLoS One. 2015 May 27;10(5):e0127397. doi: 10.1371/journal.pone.0127397. eCollection 2015. PLoS One. 2015. PMID: 26016986 Free PMC article.
-
LXR agonist improves peripheral neuropathy and modifies PNS immune cells in aged mice.J Neuroinflammation. 2022 Feb 26;19(1):57. doi: 10.1186/s12974-022-02423-z. J Neuroinflammation. 2022. PMID: 35219337 Free PMC article.
-
Immune cells contribute to myelin degeneration and axonopathic changes in mice overexpressing proteolipid protein in oligodendrocytes.J Neurosci. 2006 Aug 2;26(31):8206-16. doi: 10.1523/JNEUROSCI.1921-06.2006. J Neurosci. 2006. PMID: 16885234 Free PMC article.
References
-
- Prineas JW: Acute idiopathic polyneuritis. Lab Invest 1972, 26:133-147 - PubMed
-
- Pollard JD, Baverstock J, McLeod JG: Class II antigen expression and inflammatory cells in the Guillain-Barré syndrome. Ann Neurol 1987, 21:337-341 - PubMed
-
- Kiefer R, Kieseier BC, Stoll G, Hartung H-P: The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol 2001, 64:109-127 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

