Coordinated induction of extracellular proteolysis systems during experimental autoimmune encephalomyelitis in mice
- PMID: 11733372
- PMCID: PMC1850601
- DOI: 10.1016/S0002-9440(10)63073-8
Coordinated induction of extracellular proteolysis systems during experimental autoimmune encephalomyelitis in mice
Abstract
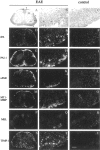
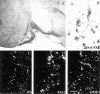
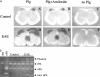
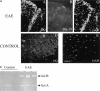
Plasminogen activators (PAs) and matrix metalloproteinases (MMPs) are considered to play an important role in the pathogenesis of multiple sclerosis. Experimental autoimmune encephalomyelitis (EAE) is widely used as an animal model of multiple sclerosis. Whereas several studies have addressed the expression of various MMPs and their inhibitors in the pathogenesis of EAE, the expression of the molecules of the PA system during EAE has not been reported previously. The present study was undertaken to investigate the expression of the molecules of the PA system (tPA, uPA, PAI-1, uPAR, LRP), as well as several members of the MMP family and their inhibitors in the course of actively induced EAE in BALB/c mice. During clinical EAE, the PA system was up-regulated in the central nervous system at several levels. Induction of expression of tPA and PAI-1 transcripts was detected in activated astrocytes in the white matter. Inflammatory cells expressed uPA receptor, uPAR. In situ zymography demonstrated the presence of increased tPA and uPA activities in the areas of the inflammatory damage. Accumulation of fibrin, fibronectin, and vitronectin immunoreactivity was seen in perivascular matrices of symptomatic animals. In addition, transcription of MT1-MMP and metalloelastase (in inflammatory cells), and TIMP-1 (in activated astrocytes) was induced during EAE. Increased gelatinolytic activity was detected at the sites of inflammatory cell accumulation by in situ zymography of fluorescently labeled gelatin; substrate gel zymography identified the up-regulated gelatinolytic activity as gelatinase B. Overall, our study demonstrates concurrent induction of PA and MMP systems during active EAE, supporting further the concept that the neuroinflammatory damage in EAE involves altered balance between multiple extracellular proteases and their inhibitors.
Figures

Similar articles
-
The plasminogen activator system: involvement in central nervous system inflammation and a potential site for therapeutic intervention.J Neuroinflammation. 2013 Oct 11;10:124. doi: 10.1186/1742-2094-10-124. J Neuroinflammation. 2013. PMID: 24120085 Free PMC article.
-
A role for the plasminogen activator system in inflammation and neurodegeneration in the central nervous system during experimental allergic encephalomyelitis.Am J Pathol. 2005 Aug;167(2):545-54. doi: 10.1016/S0002-9440(10)62996-3. Am J Pathol. 2005. PMID: 16049338 Free PMC article.
-
Expression and localization of extracellular matrix-degrading proteinases and their inhibitors in the bovine mammary gland during development, function, and involution.J Dairy Sci. 2007 Feb;90(2):740-8. doi: 10.3168/jds.S0022-0302(07)71558-8. J Dairy Sci. 2007. PMID: 17235151
-
Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system.J Neuroimmunol. 1999 Feb 1;94(1-2):1-14. doi: 10.1016/s0165-5728(98)00241-0. J Neuroimmunol. 1999. PMID: 10376931 Review.
-
Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis.Arterioscler Thromb Vasc Biol. 2001 Jul;21(7):1104-17. doi: 10.1161/hq0701.093685. Arterioscler Thromb Vasc Biol. 2001. PMID: 11451738 Review.
Cited by
-
Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse.PLoS One. 2010 Feb 3;5(2):e9027. doi: 10.1371/journal.pone.0009027. PLoS One. 2010. PMID: 20140248 Free PMC article.
-
Blocking PDGF-CC signaling ameliorates multiple sclerosis-like neuroinflammation by inhibiting disruption of the blood-brain barrier.Sci Rep. 2020 Dec 24;10(1):22383. doi: 10.1038/s41598-020-79598-z. Sci Rep. 2020. PMID: 33361796 Free PMC article.
-
Molecular imaging of the urokinase plasminogen activator receptor: opportunities beyond cancer.EJNMMI Res. 2020 Jul 28;10(1):87. doi: 10.1186/s13550-020-00673-7. EJNMMI Res. 2020. PMID: 32725278 Free PMC article. Review.
-
Roles of the tissue-type plasminogen activator in immune response.Cell Immunol. 2022 Jan;371:104451. doi: 10.1016/j.cellimm.2021.104451. Epub 2021 Nov 6. Cell Immunol. 2022. PMID: 34781155 Free PMC article. Review.
-
The Role of TLR4 and Fyn Interaction on Lipopolysaccharide-Stimulated PAI-1 Expression in Astrocytes.Mol Neurobiol. 2015 Aug;52(1):8-25. doi: 10.1007/s12035-014-8837-z. Epub 2014 Aug 9. Mol Neurobiol. 2015. PMID: 25106729
References
-
- Werb Z: ECM and cell surface proteolysis: regulating cellular ecology. Cell 1997, 91:439-442 - PubMed
-
- Matrisian LM, Hogan BLM: Growth factor-regulated proteases and extracellular matrix remodeling during mammalian development. Curr Top Dev Biol 1990, 24:219-257 - PubMed
-
- Birkedal-Hansen H: Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 1995, 7:728-735 - PubMed
-
- Andreasen PA, Kjoller L, Christensen L, Duffy MJ: The urokinase-type plasminogen activator in cancer metastasis: a review. Int J Cancer 1997, 72:1-22 - PubMed
-
- Jones JL, Walker RA: Control of matrix metalloproteinase activity in cancer. J Pathol 1997, 183:377-379 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

