Immunological characterization of human vaginal xenografts in immunocompromised mice: development of a small animal model for the study of human immunodeficiency virus-1 infection
- PMID: 11733382
- PMCID: PMC1850585
- DOI: 10.1016/S0002-9440(10)63083-0
Immunological characterization of human vaginal xenografts in immunocompromised mice: development of a small animal model for the study of human immunodeficiency virus-1 infection
Abstract
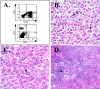
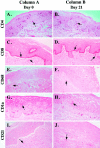
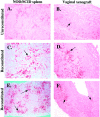
A small animal model for the in vivo study of human immunodeficiency virus-1 and other fastidious infectious agents in human host target tissues is critical for the advancement of therapeutic and preventative strategies. Our laboratory has developed a human vaginal xenograft model that histologically recapitulates features of the human vaginal epithelial barrier. Vaginal xenografts were surgically implanted into C.B.-Igh-1(b)/IcrTac-Prkdc(scid) (SCID) and NOD/LtSz-scid/scid (NOD/SCID) mice, with and without human peripheral blood mononuclear cell reconstitution. Immunohistochemical staining of vaginal xenografts demonstrated that in the SCID strain healed vaginal xenografts did not retain intrinsic human immune cells at baseline levels, whereas the NOD/SCID strain supported retention of intrinsic human immune cell populations within the xenografts for at least 2 months after engraftment. In peripheral blood mononuclear cell-reconstituted NOD/SCID mice with vaginal xenografts, flow cytometric analyses detected human immune cell populations in the peripheral blood and immunohistochemical methods detected infiltration of human CD45+ cells in the mouse spleens and vaginal xenografts for at least 2 months after reconstitution. This optimized NOD/SCID human vaginal xenograft model may provide a unique small animal in vivo system for the study of human immunodeficiency virus-1 transmission and infection.
Figures





Similar articles
-
Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice.Am J Pathol. 1995 Apr;146(4):888-902. Am J Pathol. 1995. PMID: 7717456 Free PMC article.
-
High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice.J Infect Dis. 1995 Oct;172(4):974-82. doi: 10.1093/infdis/172.4.974. J Infect Dis. 1995. PMID: 7561218
-
Multilineage engraftment in NOD/LtSz-scid/scid mice from mobilized human CD34+ peripheral blood progenitor cells.Biol Blood Marrow Transplant. 1997 Nov;3(5):236-46. Biol Blood Marrow Transplant. 1997. PMID: 9450918
-
Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection.J Reprod Immunol. 2011 Mar;88(2):195-203. doi: 10.1016/j.jri.2010.11.005. Epub 2011 Jan 21. J Reprod Immunol. 2011. PMID: 21256601 Free PMC article. Review.
-
Human neural xenografts: progress in developing an in-vivo model to study human immunodeficiency virus (HIV) and human cytomegalovirus (HCMV) infection.Adv Neuroimmunol. 1994;4(3):257-60. doi: 10.1016/s0960-5428(06)80264-0. Adv Neuroimmunol. 1994. PMID: 7874392 Review.
Cited by
-
Humanized mouse models of genetic immune disorders and hematological malignancies.Biochem Pharmacol. 2020 Apr;174:113671. doi: 10.1016/j.bcp.2019.113671. Epub 2019 Oct 18. Biochem Pharmacol. 2020. PMID: 31634456 Free PMC article. Review.
-
Differentiation of xenografted human fetal lung parenchyma.Early Hum Dev. 2008 Mar;84(3):181-93. doi: 10.1016/j.earlhumdev.2007.04.002. Epub 2007 Jun 6. Early Hum Dev. 2008. PMID: 17555893 Free PMC article.
References
-
- Orga PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR (editors): Mucosal Immunology. San Diego, Academic Press, 1999, pp 963–989
-
- Stanberry LR, Bernstein DI (editors): Sexually Transmitted Diseases: Vaccines, Prevention and Control. San Diego, Academic Press, 2000, pp 57–122
-
- Yeaman GR, White HD, Howell A, Prabhala R, Wira CR: The mucosal immune system in the human female reproductive tract: potential insights into the heterosexual transmission of HIV. AIDS Res Hum Retroviruses 1998, 14(Suppl 1):S57-S62 - PubMed
-
- Miller CJ, McGhee JR, Gardner MB: Mucosal immunity, HIV transmission, and AIDS. Lab Invest 1992, 68:129-145 - PubMed
-
- Miller CJ: Host and viral factors influencing heterosexual HIV transmission. Rev Reprod 1998, 3:42-51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

