Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice
- PMID: 11733384
- PMCID: PMC1850603
- DOI: 10.1016/S0002-9440(10)63085-4
Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice
Abstract
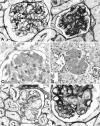
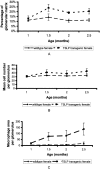
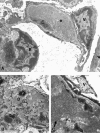
Mixed cryoglobulins are complexes of immunoglobulins that reversibly precipitate in the cold and lead to a systemic disease in humans. Renal involvement usually manifests as a membranoproliferative glomerulonephritis with marked monocyte infiltration and, at times, intracapillary thrombi. Thymic stromal lymphopoietin (TSLP) is a recently cloned cytokine that supports differentiation and long-term growth of B cells. Here we report that TSLP overexpression in mice results in the development of mixed cryoglobulins, with renal involvement closely resembling cryoglobulinemic glomerulonephritis as it occurs in humans. One hundred twenty-three mice were sacrificed at monthly intervals, with at least five TSLP transgenic mice and five controls in each group. Blood, kidneys, spleen, liver, lung, and ear were collected and studied by routine microscopy, immunofluorescence, immunohistochemistry, and electron microscopy. TSLP transgenic animals developed polyclonal mixed cryoglobulinemia (type III) and a systemic inflammatory disease involving the kidney, spleen, liver, lung, and ears. Renal involvement was of a membranoproliferative type demonstrating thickened capillary walls with cellular interposition and double contours of the basement membrane, expansion of the mesangium because of increased matrix and accumulation of immune-deposits, subendothelial immune-deposits, focal occlusion of capillary loops, and monocyte/macrophage influx. In contrast to the severe glomerular lesions, the tubulointerstitium was not involved in the disease process. The renal lesions and the disease course were more severe in females when compared to males. We describe a mouse strain in which a B-cell-promoting cytokine leads to formation of large amounts of mixed cryoglobulins and a systemic inflammatory injury that resembles important aspects of human cryoglobulinemia. This is the first reproducible mouse model of renal involvement in mixed cryoglobulinemia, which enables detailed studies of a membranoproliferative pattern of glomerular injury.
Figures





Similar articles
-
Macrophages are essential contributors to kidney injury in murine cryoglobulinemic membranoproliferative glomerulonephritis.Kidney Int. 2011 Nov;80(9):946-958. doi: 10.1038/ki.2011.249. Epub 2011 Aug 3. Kidney Int. 2011. PMID: 21814168
-
Overexpression of complement inhibitor Crry does not prevent cryoglobulin-associated membranoproliferative glomerulonephritis.Kidney Int. 2004 Apr;65(4):1214-23. doi: 10.1111/j.1523-1755.2004.00495.x. Kidney Int. 2004. PMID: 15086460
-
Oral interferon-alpha treatment of mice with cryoglobulinemic glomerulonephritis.Am J Kidney Dis. 2002 Apr;39(4):876-88. doi: 10.1053/ajkd.2002.32011. Am J Kidney Dis. 2002. PMID: 11920357
-
Heat-insoluble cryoglobulin in a patient with essential type II cryoglobulinemia and cryoglobulin-occlusive membranoproliferative glomerulonephritis: case report and literature review.Clin Chim Acta. 2009 Aug;406(1-2):170-3. doi: 10.1016/j.cca.2009.05.013. Epub 2009 May 20. Clin Chim Acta. 2009. PMID: 19463798 Review.
-
HCV-negative mixed cryoglobulinemia and kidney involvement: in-depth review on physiopathological and histological bases.Clin Exp Med. 2018 Nov;18(4):465-471. doi: 10.1007/s10238-018-0514-5. Epub 2018 Jun 28. Clin Exp Med. 2018. PMID: 29956004 Review.
Cited by
-
BTBR Ob/Ob mutant mice model progressive diabetic nephropathy.J Am Soc Nephrol. 2010 Sep;21(9):1533-42. doi: 10.1681/ASN.2009121290. Epub 2010 Jul 15. J Am Soc Nephrol. 2010. PMID: 20634301 Free PMC article.
-
WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity.J Exp Med. 2011 Sep 26;208(10):2033-42. doi: 10.1084/jem.20110200. Epub 2011 Aug 29. J Exp Med. 2011. PMID: 21875954 Free PMC article.
-
The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond.J Leukoc Biol. 2012 Jun;91(6):877-86. doi: 10.1189/jlb.1211622. Epub 2012 Mar 21. J Leukoc Biol. 2012. PMID: 22442496 Free PMC article. Review.
-
Thymic stromal lymphopoietin transgenic mice develop cryoglobulinemia and hepatitis with similarities to human hepatitis C liver disease.Am J Pathol. 2007 Mar;170(3):981-9. doi: 10.2353/ajpath.2007.060474. Am J Pathol. 2007. PMID: 17322382 Free PMC article.
-
Deletion of activating Fcgamma receptors does not confer protection in murine cryoglobulinemia-associated membranoproliferative glomerulonephritis.Am J Pathol. 2009 Jul;175(1):107-18. doi: 10.2353/ajpath.2009.081159. Epub 2009 Jun 15. Am J Pathol. 2009. PMID: 19528347 Free PMC article.
References
-
- Ramos-Casals M, Trejo O, Garcia-Carrasco M, Cervera R, Font J: Mixed cryoglobulinemia: new concepts. Lupus 2000, 9:83-91 - PubMed
-
- Cacoub P, Renou C, Rosenthal E, Cohen P, Loury I, Loustaud-Ratti V, Yamamoto AM, Camproux AC, Hausfater P, Musset L, Veyssier P, Raguin G, Piette JC: Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine 2000, 79:47-56 - PubMed
-
- Sinico RA, Fornasieri A, D’Amico G: Renal manifestations associated with hepatitis C virus. Ann Med Interne 2000, 151:41-45 - PubMed
-
- Kallemuchikkal U, Gorevic PD: Evaluation of cryoglobulins. Arch Pathol Lab Med 1999, 123:119-125 - PubMed
-
- Dispenzieri A, Gorevic PD: Cryoglobulinemia. Hematol Oncol Clin North Am 1999, 13:1315-1349 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

