A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae
- PMID: 11733545
- PMCID: PMC2150915
- DOI: 10.1083/jcb.200107028
A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae
Abstract
In vivo time-lapse microscopy reveals that the number of peroxisomes in Saccharomyces cerevisiae cells is fairly constant and that a subset of the organelles are targeted and segregated to the bud in a highly ordered, vectorial process. The dynamin-like protein Vps1p controls the number of peroxisomes, since in a vps1Delta mutant only one or two giant peroxisomes remain. Analogous to the function of other dynamin-related proteins, Vps1p may be involved in a membrane fission event that is required for the regulation of peroxisome abundance. We found that efficient segregation of peroxisomes from mother to bud is dependent on the actin cytoskeleton, and active movement of peroxisomes along actin filaments is driven by the class V myosin motor protein, Myo2p: (a) peroxisomal dynamics always paralleled the polarity of the actin cytoskeleton, (b) double labeling of peroxisomes and actin cables revealed a close association between both, (c) depolymerization of the actin cytoskeleton abolished all peroxisomal movements, and (d) in cells containing thermosensitive alleles of MYO2, all peroxisome movement immediately stopped at the nonpermissive temperature. In addition, time-lapse videos showing peroxisome movement in wild-type and vps1Delta cells suggest the existence of various levels of control involved in the partitioning of peroxisomes.
Figures



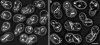



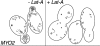
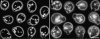
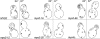
References
-
- Beach, D.L., J. Thibodeaux, P. Maddox, E. Yeh, and K. Bloom. 2000. The role of the proteins Kar9 and Myo2 in orienting the mitotic spindle of budding yeast. Curr. Biol. 10:1497–1506. - PubMed
-
- Bobola, N., R.P. Jansen, T.H. Shin, and K. Nasmith. 1996. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell. 84:699–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

