Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin
- PMID: 11733557
- PMCID: PMC200981
- DOI: 10.1172/JCI12849
Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin
Abstract
NPHS2 was recently identified as a gene whose mutations cause autosomal recessive steroid-resistant nephrotic syndrome. Its product, podocin, is a new member of the stomatin family, which consists of hairpin-like integral membrane proteins with intracellular NH(2)- and COOH-termini. Podocin is expressed in glomerular podocytes, but its subcellular distribution and interaction with other proteins are unknown. Here we show, by immunoelectron microscopy, that podocin localizes to the podocyte foot process membrane, at the insertion site of the slit diaphragm. Podocin accumulates in an oligomeric form in lipid rafts of the slit diaphragm. Moreover, GST pull-down experiments reveal that podocin associates via its COOH-terminal domain with CD2AP, a cytoplasmic binding partner of nephrin, and with nephrin itself. That podocin interacts with CD2AP and nephrin in vivo is shown by coimmunoprecipitation of these proteins from glomerular extracts. Furthermore, in vitro studies reveal direct interaction of podocin and CD2AP. Hence, as with the erythrocyte lipid raft protein stomatin, podocin is present in high-order oligomers and may serve a scaffolding function. We postulate that podocin serves in the structural organization of the slit diaphragm and the regulation of its filtration function.
Figures
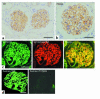
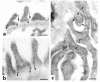
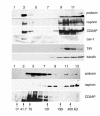
Comment in
-
Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis.J Clin Invest. 2001 Dec;108(11):1583-7. doi: 10.1172/JCI14629. J Clin Invest. 2001. PMID: 11733553 Free PMC article. No abstract available.
References
-
- Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 1995;192:385–397. - PubMed
-
- Drumond MC, Deen WM. Structural determinants of glomerular hydraulic permeability. Am J Physiol. 1994;266:F1–F12. - PubMed
-
- Smoyer WE, Mundel P. Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med. 1998;76:172–183. - PubMed
-
- Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

