Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation
- PMID: 11733571
- PMCID: PMC2193528
- DOI: 10.1084/jem.194.11.1561
Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation
Abstract
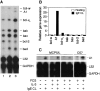
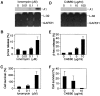
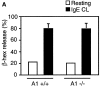
Mast cells reside in tissues, where upon activation through the high-affinity-IgE-receptor (FcepsilonRI) they degranulate and orchestrate the allergic reaction. Mast cells survive this activation and can thus be reactivated. In this study we demonstrate that this process depends on the pro-survival gene A1. Activation of mast cells through FcepsilonRI resulted in degranulation, strong induction of A1 mRNA and protein, and cell survival. In contrast, A1-deficient mast cells released granule mediators similar to the wild-type control, but the cells did not survive an allergic activation. Furthermore, A1(-/-) mice that had been sensitized and provoked with allergen exhibited a lower number of mast cell compared with littermate controls. The induction of A1 was dependent on calcium, as EDTA prevented A1 expression. The calcium ionophore, ionomycin, induced A1 expression and mast cell survival, whereas compound 48/80, a well-known mast cell secretagogue, did not. This study uncovers the importance of A1 for mast cell survival in allergic reactions, and it proposes A1 as a potential target for the treatment of allergic diseases.
Figures








Similar articles
-
NFAT but not NF-kappaB is critical for transcriptional induction of the prosurvival gene A1 after IgE receptor activation in mast cells.Blood. 2008 Mar 15;111(6):3081-9. doi: 10.1182/blood-2006-10-053371. Epub 2008 Jan 8. Blood. 2008. PMID: 18182578 Free PMC article.
-
IL-3 but not monomeric IgE regulates FcεRI levels and cell survival in primary human basophils.Cell Death Dis. 2018 May 1;9(5):510. doi: 10.1038/s41419-018-0526-9. Cell Death Dis. 2018. PMID: 29724998 Free PMC article.
-
FcepsilonRI aggregation promotes survival of connective tissue-like mast cells but not mucosal-like mast cells.J Immunol. 2007 Apr 1;178(7):4177-83. doi: 10.4049/jimmunol.178.7.4177. J Immunol. 2007. PMID: 17371974
-
News in Cellular Allergology: A Review of the Human Mast Cell and Basophil Granulocyte Literature from January 2013 to May 2015.Int Arch Allergy Immunol. 2015;168(4):253-62. doi: 10.1159/000443960. Epub 2016 Feb 20. Int Arch Allergy Immunol. 2015. PMID: 26895271 Review.
-
The role of c-Kit and its ligand, stem cell factor, in mast cell apoptosis.Int Arch Allergy Immunol. 1995 May-Jun;107(1-3):136-8. doi: 10.1159/000236955. Int Arch Allergy Immunol. 1995. PMID: 7542059 Review.
Cited by
-
Regulation of A1 by OX40 contributes to CD8(+) T cell survival and anti-tumor activity.PLoS One. 2013 Aug 1;8(8):e70635. doi: 10.1371/journal.pone.0070635. Print 2013. PLoS One. 2013. PMID: 23936461 Free PMC article.
-
Anti-apoptotic A1 is not essential for lymphoma development in Eµ-Myc mice but helps sustain transplanted Eµ-Myc tumour cells.Cell Death Differ. 2018 Mar;25(4):797-808. doi: 10.1038/s41418-017-0045-8. Epub 2018 Jan 16. Cell Death Differ. 2018. PMID: 29339775 Free PMC article.
-
Absence of pro-survival A1 has no impact on inflammatory cell survival in vivo during acute lung inflammation and peritonitis.Cell Death Differ. 2022 Jan;29(1):96-104. doi: 10.1038/s41418-021-00839-3. Epub 2021 Jul 24. Cell Death Differ. 2022. PMID: 34304242 Free PMC article.
-
Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases.EMBO J. 2011 Aug 23;30(18):3667-83. doi: 10.1038/emboj.2011.307. EMBO J. 2011. PMID: 21863020 Free PMC article. Review.
-
Pro-apoptotic Bax is the major and Bak an auxiliary effector in cytokine deprivation-induced mast cell apoptosis.Cell Death Dis. 2010 May 13;1(5):e43. doi: 10.1038/cddis.2010.20. Cell Death Dis. 2010. PMID: 21364649 Free PMC article.
References
-
- Metcalfe, D.D., D. Baram, and Y.A. Mekori. 1997. Mast cells. Physiol. Rev. 77:1033–1079. - PubMed
-
- Nilsson, G., J.J. Costa, and D.D. Metcalfe. 1999. Mast cells and basophils. Inflammation: Basic Principles and Clinical Correlates. J.I. Gallin and R. Snyderman, editors. Lippincott-Raven publications, Philadelphia, PA. 97–117.
-
- Galli, S.J. 2000. Allergy. Curr. Biol. 10:R93–R95. - PubMed
-
- Otsuka, H., J. Denburg, J. Dolovich, D. Hitch, P. Lapp, R.S. Rajan, J. Bienenstock, and D. Befus. 1985. Heterogeneity of metachromatic cells in human nose: Significance of mucosal mast cells. J. Allergy Clin. Immunol. 76:695–702. - PubMed
-
- Wardlaw, A.J., S. Dunnette, G.J. Gleich, J.V. Collins, and A.B. Kay. 1988. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am. Rev. Respir. Dis. 137:62–69. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

