Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis
- PMID: 11733573
- PMCID: PMC2193524
- DOI: 10.1084/jem.194.11.1583
Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis
Abstract
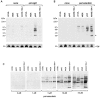
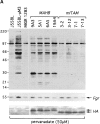
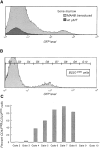
Signal transduction through the B cell antigen receptor (BCR) is determined by a balance of positive and negative regulators. This balance is shifted by aggregation that results from binding to extracellular ligand. Aggregation of the BCR is necessary for eliciting negative selection or activation by BCR-expressing B cells. However, ligand-independent signaling through intermediate and mature forms of the BCR has been postulated to regulate B cell development and peripheral homeostasis. To address the importance of ligand-independent BCR signaling functions and their regulation during B cell development, we have designed a model that allows us to isolate the basal signaling functions of immunoglobulin (Ig)alpha/Igbeta-containing BCR complexes from those that are dependent upon ligand-mediated aggregation. In vivo, we find that basal signaling is sufficient to facilitate pro-B --> pre-B cell transition and to generate immature/mature peripheral B cells. The ability to generate basal signals and to drive developmental progression were both dependent on plasma membrane association of Igalpha/Igbeta complexes and intact immunoregulatory tyrosine activation motifs (ITAM), thereby establishing a correlation between these processes. We believe that these studies are the first to directly demonstrate biologically relevant basal signaling through the BCR where the ability to interact with both conventional as well as nonconventional extracellular ligands is eliminated.
Figures




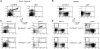



References
-
- Bromley, S.K., W.R. Burack, K.G. Johnson, K. Somersalo, T.N. Sims, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375–396. - PubMed
-
- Monks, C.R., B.A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 395:82–86. - PubMed
-
- Delon, J., and R.N. Germain. 2000. Information transfer at the immunological synapse. Curr. Biol. 10:R923–R933. - PubMed
-
- Batista, F.D., D. Iber, and M.S. Neuberger. 2001. B cells acquire antigen from target cells after synapse formation. Nature. 411:489–494. - PubMed
-
- Korade-Mirnics, Z., and S.J. Corey. 2000. Src kinase-mediated signaling in leukocytes. J. Leukoc. Biol. 68:603–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

