Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function
- PMID: 11733581
- PMCID: PMC2193535
- DOI: 10.1084/jem.194.11.1675
Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function
Abstract
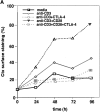
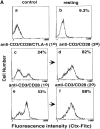
Coreceptors CD28 and cytotoxic T lymphocyte antigen (CTLA)-4 have opposing effects on TcR/CD3 activation of T cells. While CD28 enhances and CTLA-4 inhibits activation, the underlying molecular basis of these effects has yet to be established. In this context, ganglioside and cholesterol enriched membrane microdomains (rafts, GEMs) serve as centers of signaling in T cells. Although CD28 can promote TcR/raft colocalization, evidence is lacking on whether the surface expression of membrane rafts can be targeted by CTLA-4 in its modulation of T cell responses. In this study, we demonstrate that both CD28 and CTLA-4 profoundly alter the surface expression of membrane rafts during T cell activation. While CD28 increased expression and the number of peripheral T cells induced to express surface rafts in response to TcR ligation, CTLA-4 potently inhibited both TcR and TcR x CD28 induced raft expression on the surface of T cells. Consistent with this, CD28 increased the presence of the linker of activated T cells (LAT) in purified membrane rafts, while CTLA-4 coligation effectively blocked this increase. Further, the reversal of the CTLA-4 block with CD3/CD28 ligation was accompanied by an increase in surface raft expression and associated LAT. Our observations demonstrate for the first time that CTLA-4 targets the release of rafts to the surface of T cells, and provides a mechanism for the opposing effects of CD28 and CTLA-4 on costimulation.
Figures







References
-
- June, C.H., J.A. Bluestone, L.M. Nadler, and C.B. Thompson. 1994. The B7 and CD28 receptor families. Immunol. Today. 15:321–331. - PubMed
-
- Chambers, C.A., and J.P. Allison. 1999. Co-stimulatory regulation of T cell function. Curr. Opin. Cell. Biol. 11:203–210. - PubMed
-
- Walunas, T.L., D.J. Lenschow, C.Y. Bakker, P.S. Linsley, G.J. Freeman, J.M. Green, C.B. Thompson, and J.A. Bluestone. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1:405–413. - PubMed
-
- Rudd, C.E. 1996. Upstream-downstream: CD28 cosignaling pathways and T cell function. Immunity. 4:527–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

