Generation of choline for acetylcholine synthesis by phospholipase D isoforms
- PMID: 11734063
- PMCID: PMC60648
- DOI: 10.1186/1471-2202-2-16
Generation of choline for acetylcholine synthesis by phospholipase D isoforms
Abstract
Background: In cholinergic neurons, the hydrolysis of phosphatidylcholine (PC) by a phospholipase D (PLD)-type enzyme generates some of the precursor choline used for the synthesis of the neurotransmitter acetylcholine (ACh). We sought to determine the molecular identity of the relevant PLD using murine basal forebrain cholinergic SN56 cells in which the expression and activity of the two PLD isoforms, PLD1 and PLD2, were experimentally modified. ACh levels were examined in cells incubated in a choline-free medium, to ensure that their ACh was synthesized entirely from intracellular choline.
Results: PLD2, but not PLD1, mRNA and protein were detected in these cells and endogenous PLD activity and ACh synthesis were stimulated by phorbol 12-myristate 13-acetate (PMA). Introduction of a PLD2 antisense oligonucleotide into the cells reduced PLD2 mRNA and protein expression by approximately 30%. The PLD2 antisense oligomer similarly reduced basal- and PMA-stimulated PLD activity and ACh levels. Overexpression of mouse PLD2 by transient transfection increased basal- (by 74%) and PMA-stimulated (by 3.2-fold) PLD activity. Moreover, PLD2 transfection increased ACh levels by 26% in the absence of PMA and by 2.1-fold in the presence of PMA. Overexpression of human PLD1 by transient transfection increased PLD activity by 4.6-fold and ACh synthesis by 2.3-fold in the presence of PMA as compared to controls.
Conclusions: These data identify PLD2 as the endogenous enzyme that hydrolyzes PC to generate choline for ACh synthesis in cholinergic cells, and indicate that in a model system choline generated by PLD1 may also be used for this purpose.
Figures
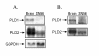


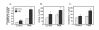
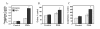
References
-
- Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–620. - PubMed
-
- Hammond SM, Altshuller YM, Sung TC, Rudge SA, Rose K, Engebrecht J, Morris AJ, Frohman MA. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J Biol Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

