The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction
- PMID: 11734628
- PMCID: PMC65005
- DOI: 10.1073/pnas.261452898
The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction
Abstract
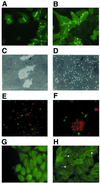
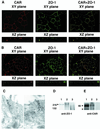
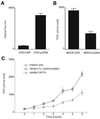
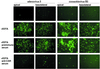
The coxsackievirus and adenovirus receptor (CAR) mediates viral attachment and infection, but its physiologic functions have not been described. In nonpolarized cells, CAR localized to homotypic intercellular contacts, mediated homotypic cell aggregation, and recruited the tight junction protein ZO-1 to sites of cell-cell contact. In polarized epithelial cells, CAR and ZO-1 colocalized to tight junctions and could be coprecipitated from cell lysates. CAR expression led to reduced passage of macromolecules and ions across cell monolayers, and soluble CAR inhibited the formation of functional tight junctions. Virus entry into polarized epithelium required disruption of tight junctions. These results indicate that CAR is a component of the tight junction and of the functional barrier to paracellular solute movement. Sequestration of CAR in tight junctions may limit virus infection across epithelial surfaces.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

