A second iron-regulatory system in yeast independent of Aft1p
- PMID: 11734641
- PMCID: PMC64680
- DOI: 10.1073/pnas.261381198
A second iron-regulatory system in yeast independent of Aft1p
Abstract
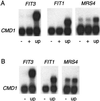
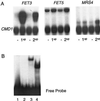
Iron homeostasis in the yeast Saccharomyces cerevisiae is regulated at the transcriptional level by Aft1p, which activates the expression of its target genes in response to low-iron conditions. The yeast genome contains a paralog of AFT1, which has been designated AFT2. To establish whether AFT1 and AFT2 have overlapping functions, a mutant containing a double aft1Deltaaft2Delta deletion was generated. Growth assays established that the single aft2Delta strain exhibited no iron-dependent phenotype. However, the double-mutant aft1Deltaaft2Delta strain was more sensitive to low-iron growth conditions than the single-mutant aft1Delta strain. A mutant allele of AFT2 (AFT2-1(up)), or overexpression of the wild-type AFT2 gene, led to partial complementation of the respiratory-deficient phenotype of the aft1Delta strain. The AFT2-1(up) allele also increased the uptake of (59)Fe in an aft1Delta strain. DNA microarrays were used to identify genes regulated by AFT2. Some of the AFT2-regulated genes are known to be regulated by Aft1p; however, AFT2-1(up)-dependent activation was independent of Aft1p. The kinetics of induction of two genes activated by the AFT2-1(up) allele are consistent with Aft2p acting as a direct transcriptional factor. Truncated forms of Aft1p and Aft2p bound to a DNA duplex containing the Aft1p binding site in vitro. The wild-type allele of AFT2 activated transcription in response to growth under low-iron conditions. Together, these data suggest that yeast has a second regulatory pathway for the iron regulon, with AFT1 and AFT2 playing partially redundant roles.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

