Fast dynamics of halophilic malate dehydrogenase and BSA measured by neutron scattering under various solvent conditions influencing protein stability
- PMID: 11734642
- PMCID: PMC64686
- DOI: 10.1073/pnas.251537298
Fast dynamics of halophilic malate dehydrogenase and BSA measured by neutron scattering under various solvent conditions influencing protein stability
Abstract
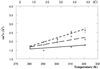
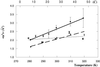
Protein thermal dynamics was evaluated by neutron scattering for halophilic malate dehydrogenase from Haloarcula marismortui (HmMalDH) and BSA under different solvent conditions. As a measure of thermal stability in each case, loss of secondary structure temperatures were determined by CD. HmMalDH requires molar salt and has different stability behavior in H(2)O, D(2)O, and in NaCl and KCl solvents. BSA remains soluble in molar NaCl. The neutron experiments provided values of mean-squared atomic fluctuations at the 0.1 ns time scale. Effective force constants, characterizing the mean resilience of the protein structure, were calculated from the variation of the mean-squared fluctuation with temperature. For HmMalDH, resilience increased progressively with increasing stability, from molar NaCl in H(2)O, via molar KCl in D(2)O, to molar NaCl in D(2)O. Surprisingly, however, the opposite was observed for BSA; its resilience is higher in H(2)O where it is less stable than in D(2)O. These results confirmed the complexity of dynamics-stability relationships in different proteins. Softer dynamics for BSA in D(2)O showed that the higher thermostability is associated with entropic fluctuations. In the halophilic protein, higher stability is associated with increased resilience showing the dominance of enthalpic terms arising from bonded interactions. From previous data, it is suggested that these are associated with hydrated ion binding stabilizing the protein in the high-salt solvent.
Figures

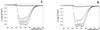


References
-
- Sohl J L, Jaswal S S, Agard D A. Nature (London) 1998;395:817–819. - PubMed
-
- Zaccai G. Science. 2000;288:1604–1607. - PubMed
-
- Bu Z, Neumann D A, Lee S H, Brown C M, Engelman D M, Han C C. J Mol Biol. 2000;301:525–536. - PubMed
-
- Bée M. Quasielastic Neutron Scattering: Principles and Applications in Solid State Chemistry, Biology and Materials Science. Philadelphia, PA: Adam Hilger; 1988.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

