Induction of platelet formation from megakaryocytoid cells by nitric oxide
- PMID: 11734646
- PMCID: PMC64703
- DOI: 10.1073/pnas.241427398
Induction of platelet formation from megakaryocytoid cells by nitric oxide
Abstract
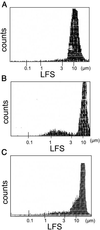
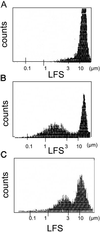
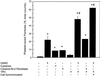
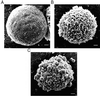
Although the growth factors that regulate megakaryocytopoiesis are well known, the molecular determinants of platelet formation from mature megakaryocytes remain poorly understood. Morphological changes in megakaryocytes associated with platelet formation and removal of senescent megakaryocytes are suggestive of an apoptotic process. Previously, we have established that nitric oxide (NO) can induce apoptosis in megakaryocytoid cell lines. To determine whether there is an association between NO-induced apoptosis and platelet production, we exposed Meg-01 cells to S-nitrosoglutathione (GSNO) with or without thrombopoeitin (TPO) pretreatment and used flow cytometry and electron microscopy to assess platelet-sized particle formation. Meg-01 cells treated with TPO alone produced few platelet-sized particles (<3% of total counts), whereas treatment with GSNO alone produced a significant percentage of platelet-sized particles (22 +/- 4% of total counts); when combined with TPO pretreatment, however, GSNO led to a marked increase in platelet-sized particle production (48 +/- 3% of total counts). Electron microscopy confirmed that Meg-01 cells treated with TPO and GSNO yielded platelet-sized particles with morphological features specific for platelet forms. The platelet-sized particle population appears to be functional, because addition of calcium, fibrinogen, and thrombin receptor-activating peptide led to aggregation. These results demonstrate that NO facilitates platelet production, thereby establishing the essential role of NO in megakaryocyte development and thrombopoiesis.
Figures


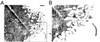

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

