Population pharmacokinetics and pharmacodynamics of oral etoposide
- PMID: 11736859
- PMCID: PMC2014597
- DOI: 10.1046/j.0306-5251.2001.01468.x
Population pharmacokinetics and pharmacodynamics of oral etoposide
Abstract
Aims: To study the population pharmacokinetics and pharmacodynamics of oral etoposide in patients with solid tumours.
Methods: A prospective, open label, cross-over, bioavailability study was performed in 50 adult patients with miscellaneous, advanced stage solid tumours, who were receiving oral (100 mg capsules) etoposide for 14 days and i.v. (50 mg) etoposide on day 1 or day 7 in randomised order during the first cycle treatment. Total and unbound etoposide concentration were assayed by h.p.l.c. Population PK parameters estimation was done by using the P-Pharm software (Simed). Haematological toxicity and tumour response were the main pharmacodynamic endpoints.
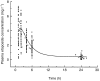
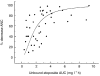
Results: Mean clearance was 1.14 l h(-1) (CV 25%). Creatinine clearance was the only covariable to significantly reduce clearance variability (residual CV 18%). (CL = 0.74 + 0.0057 CLCR; r(2) = 0.32). Mean bioavailability was 45% (CV 22%) and mean protein binding 91.5% (CV 5%). Exposure to free, pharmacologically active etoposide (free AUC p.o.) was highly variable (mean value 2.8 mg l(-1) h; CV 64%; range 0.4-9.5). It decreased with increased creatinine clearance and increased with age which accounted for 9% of the CV. Mean free AUC p.o. was the best predictor of neutropenia. Free AUC50 (exposure producing a 50% reduction in absolute neutrophil count) was 1.80 mg l(-1) h. In patients with lung cancer, the free AUC p.o. was higher in the two patients with responsive tumour (5.9 mg l(-1) h) than in patients with stable (2.1 mg l-1 h) or progressive disease (2.3 mg l-1 h) (P = 0.01).
Conclusions: Exposure to free etoposide during prolonged oral treatment is highly variable and is the main determinant of pharmacodynamic effects. The population PK model based on creatinine clearance is poorly predictive of exposure. Therapeutic drug monitoring would be necessary for dose individualization or to study the relationship between exposure and antitumour effect.
Figures

References
-
- Bruno R, Hille D, Riva A, et al. Population pharmacokinetics/ Pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:511–519. - PubMed
-
- McLeod HL, Graham MA, Aamdal S, Setanoians A, Groot Y, Lund B. Phase I pharmacokinetics and limited sample strategies for the bioreductive alkylating drug EO9. Eur J Cancer. 1996;32A:1518–1522. - PubMed
-
- Launay MC, Iliadis MC, Lacarelle B. Population pharmacokinetics of mitoxantrone performed by a NONMEM method. J Pharm Sci. 1989;78:877–880. - PubMed
-
- Wade JR, Kelman AW, Kerr DJ, Robert J, Whiting B. Variability in the pharmacokinetics of epirubicin: a population analysis. Cancer Chemother Pharmacol. 1992;29:391–395. - PubMed
-
- Nguyen L, Chatelut E, Chevreau C, et al. Population pharmacokinetics of total and unbound etoposide. Cancer Chemother Pharmacol. 1998;41:125–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

