Population pharmacokinetics of the new antimalarial agent tafenoquine in Thai soldiers
- PMID: 11736877
- PMCID: PMC2014562
- DOI: 10.1046/j.0306-5251.2001.01482.x
Population pharmacokinetics of the new antimalarial agent tafenoquine in Thai soldiers
Abstract
Aims: To describe the population pharmacokinetics of tafenoquine in healthy volunteers after receiving tafenoquine for malaria prophylaxis.
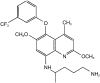
Methods: The population consisted of 135 male Thai soldiers (mean age 28.9 years; weight 60.3 kg). All soldiers were presumptively treated with artesunate for 3 days plus doxycycline for 7 days to remove any pre-existing malaria infections. After the treatment regime, 104 soldiers (drug group) received a loading dose of 400 mg tafenoquine base daily for 3 days followed by 400 mg tafenoquine monthly for 5 consecutive months. In the placebo group, 31 soldiers were infected with malaria during the study period. They were re-treated with artesunate for 3 days plus doxycycline for 7 days followed by a loading dose of 400 mg tafenoquine daily for 3 days and then 400 mg tafenoquine weekly for prophylaxis. Blood samples were randomly collected from each soldier on monthly and weekly prophylaxis. Plasma tafenoquine concentrations were measured by h.p.l.c. Population pharmacokinetic modelling was performed using NONMEM.
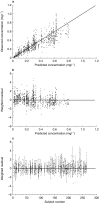
Results: A one-compartment model was found best to describe the pharmacokinetics of tafenoquine after oral administration. Age and weight influenced volume of distribution (V/F), and subjects who contracted malaria had higher clearance (CL/F), but none of these factors was considered to have sufficient impact to warrant change in dosing. The population estimates of the first-order absorption rate constant (Ka), CL/F and V/F were 0.694 h(-1), 3.20 l h(-1) and 1820 l, respectively. The intersubject variability in these parameters (coefficient of variation, CV%) was 61.2%, 25.3% and 14.8%, respectively. The absorption and elimination half-lives were 1.0 h and 16.4 days, respectively. The residual (unexplained) variability was 17.9%.
Conclusions: The population pharmacokinetics of orally administered tafenoquine have been determined in Thai soldiers under field conditions. This information, together with its known potent antimalarial activity, portends well for the application of tafenoquine as a useful prophylactic drug or for short-term radical treatment of vivax malaria.
Figures

References
-
- World Health Organization. Practical chemotherapy of malaria. World Health Organ Tech Rep Series. 1990;805:1–141. - PubMed
-
- Anonymous. Towards a malaria vaccine [editorial] Lancet. 1992;339:586–587. - PubMed
-
- Brueckner RP, Lasseter KC, Lin ET, Schuster BG. First-time-in-humans safety and pharmacokinetics of WR 238 605, a new antimalarial. Am J Trop Med Hyg. 1998;58:645–649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

