Golgi matrix proteins interact with p24 cargo receptors and aid their efficient retention in the Golgi apparatus
- PMID: 11739402
- PMCID: PMC2150891
- DOI: 10.1083/jcb.200108102
Golgi matrix proteins interact with p24 cargo receptors and aid their efficient retention in the Golgi apparatus
Abstract
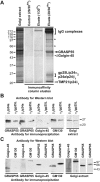
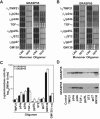
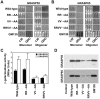
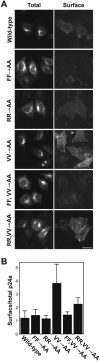
The Golgi apparatus is a highly complex organelle comprised of a stack of cisternal membranes on the secretory pathway from the ER to the cell surface. This structure is maintained by an exoskeleton or Golgi matrix constructed from a family of coiled-coil proteins, the golgins, and other peripheral membrane components such as GRASP55 and GRASP65. Here we find that TMP21, p24a, and gp25L, members of the p24 cargo receptor family, are present in complexes with GRASP55 and GRASP65 in vivo. GRASPs interact directly with the cytoplasmic domains of specific p24 cargo receptors depending on their oligomeric state, and mutation of the GRASP binding site in the cytoplasmic tail of one of these, p24a, results in it being transported to the cell surface. These results suggest that one function of the Golgi matrix is to aid efficient retention or sequestration of p24 cargo receptors and other membrane proteins in the Golgi apparatus.
Figures
References
-
- Allan, B.B., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 289:444–448. - PubMed
-
- Barr, F.A., M. Puype, J. Vandekerckhove, and G. Warren. 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 91:253–262. - PubMed
-
- Blum, R., D.J. Stephens, and I. Schultz. 2000. Lumenal targeted GFP, used as a marker of soluble cargo, visualises rapid ERGIC to Golgi traffic by a tubulo-vesicular network. J. Cell Sci. 113:3151–3159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

