Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors
- PMID: 11739587
- PMCID: PMC6763054
- DOI: 10.1523/JNEUROSCI.21-24-09792.2001
Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors
Abstract
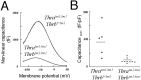
The deafness caused by early onset hypothyroidism indicates that thyroid hormone is essential for the development of hearing. We investigated the underlying roles of the TRalpha1 and TRbeta thyroid hormone receptors in the auditory system using receptor-deficient mice. TRalpha1 and TRbeta, which act as hormone-activated transcription factors, are encoded by the Thra and Thrb genes, respectively, and both are expressed in the developing cochlea. TRbeta is required for hearing because TRbeta-deficient (Thrb(tm1/tm1)) mice have a defective auditory-evoked brainstem response and retarded expression of a potassium current (I(K,f)) in the cochlear inner hair cells. Here, we show that although TRalpha1 is individually dispensable, TRalpha1 and TRbeta synergistically control an extended array of functions in postnatal cochlear development. Compared with Thrb(tm1/tm1) mice, the deletion of all TRs in Thra(tm1/tm1)Thrb(tm1/tm1) mice produces exacerbated and novel phenotypes, including delayed differentiation of the sensory epithelium, malformation of the tectorial membrane, impairment of electromechanical transduction in outer hair cells, and a low endocochlear potential. The induction of I(K,f) in inner hair cells was not markedly more retarded than in Thrb(tm1/tm1) mice, suggesting that this feature of hair cell maturation is primarily TRbeta-dependent. These results indicate that distinct pathways mediated by TRbeta alone or by TRbeta and TRalpha1 together facilitate control over an extended range of functions during the maturation of the cochlea.
Figures



Similar articles
-
Suppression of the deafness and thyroid dysfunction in Thrb-null mice by an independent mutation in the Thra thyroid hormone receptor alpha gene.Hum Mol Genet. 2001 Nov 1;10(23):2701-8. doi: 10.1093/hmg/10.23.2701. Hum Mol Genet. 2001. PMID: 11726557
-
Thyroid hormone receptor beta-dependent expression of a potassium conductance in inner hair cells at the onset of hearing.Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15758-62. doi: 10.1073/pnas.95.26.15758. Proc Natl Acad Sci U S A. 1998. PMID: 9861043 Free PMC article.
-
Autonomous functions of murine thyroid hormone receptor TRα and TRβ in cochlear hair cells.Mol Cell Endocrinol. 2014 Jan 25;382(1):26-37. doi: 10.1016/j.mce.2013.08.025. Epub 2013 Sep 6. Mol Cell Endocrinol. 2014. PMID: 24012852
-
Thyroid hormone receptors in brain development and function.Nat Clin Pract Endocrinol Metab. 2007 Mar;3(3):249-59. doi: 10.1038/ncpendmet0424. Nat Clin Pract Endocrinol Metab. 2007. PMID: 17315033 Review.
-
The effect of acoustic trauma on the tectorial membrane, stereocilia, and hearing sensitivity: possible mechanisms underlying damage, recovery, and protection.Scand Audiol Suppl. 1988;27:1-45. Scand Audiol Suppl. 1988. PMID: 3043645 Review.
Cited by
-
Thyroid hormone increases fibroblast growth factor receptor expression and disrupts cell mechanics in the developing organ of corti.BMC Dev Biol. 2013 Feb 9;13:6. doi: 10.1186/1471-213X-13-6. BMC Dev Biol. 2013. PMID: 23394545 Free PMC article.
-
Thyroid hormone action in the absence of thyroid hormone receptor DNA-binding in vivo.J Clin Invest. 2003 Aug;112(4):588-97. doi: 10.1172/JCI18377. J Clin Invest. 2003. PMID: 12925699 Free PMC article.
-
The timecourse of apoptotic cell death during postnatal remodeling of the mouse cochlea and its premature onset by triiodothyronine (T3).Mol Cell Endocrinol. 2015 May 15;407:1-8. doi: 10.1016/j.mce.2015.02.025. Epub 2015 Feb 28. Mol Cell Endocrinol. 2015. PMID: 25737207 Free PMC article.
-
Thyroxine Regulates the Opening of the Organ of Corti through Affecting P-Cadherin and Acetylated Microtubule.Int J Mol Sci. 2022 Nov 1;23(21):13339. doi: 10.3390/ijms232113339. Int J Mol Sci. 2022. PMID: 36362134 Free PMC article.
-
Deafness in TRbeta mutants is caused by malformation of the tectorial membrane.J Neurosci. 2009 Feb 25;29(8):2581-7. doi: 10.1523/JNEUROSCI.3557-08.2009. J Neurosci. 2009. PMID: 19244534 Free PMC article.
References
-
- Brucker-Davis F, Skarulis MC, Pikus A, Ishizawar D, Mastroianni M-A, Koby M, Weintraub BD. Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone (RTH). J Clin Endocrinol Metab. 1996;81:2768–2772. - PubMed
-
- Dallos P, Popper A, Fay R. The cochlea. Springer; New York: 1996.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
