Role of calcium, glutamate neurotransmission, and nitric oxide in spreading acidification and depression in the cerebellar cortex
- PMID: 11739595
- PMCID: PMC6763051
- DOI: 10.1523/JNEUROSCI.21-24-09877.2001
Role of calcium, glutamate neurotransmission, and nitric oxide in spreading acidification and depression in the cerebellar cortex
Abstract
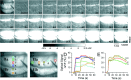
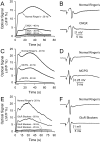
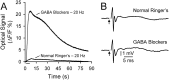
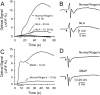
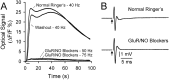
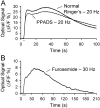
This study investigated the mechanisms underlying the recently reported fast spreading acidification and transient depression in the cerebellar cortex in vivo. Spreading acidification was evoked by surface stimulation in the rat and mouse cerebellar cortex stained with the pH-sensitive dye neutral red and monitored using epifluorescent imaging. The probability of evoking spreading acidification was dependent on stimulation parameters; greater frequency and/or greater amplitude were more effective. Although activation of the parallel fibers defined the geometry of the spread, their activation alone was not sufficient, because blocking synaptic transmission with low Ca(2+) prevented spreading acidification. Increased postsynaptic excitability was also a major factor. Application of either AMPA or metabotropic glutamate receptor antagonists reduced the likelihood of evoking spreading acidification, but stronger stimulation intensities were still effective. Conversely, superfusion with GABA receptor antagonists decreased the threshold for evoking spreading acidification. Blocking nitric oxide synthase (NOS) increased the threshold for spreading acidification, and nitric oxide donors lowered the threshold. However, spreading acidification could be evoked in neuronal NOS-deficient mice (B6;129S-Nos1(tm1plh)). The depression in cortical excitability that accompanies spreading acidification occurred in the presence of AMPA and metabotropic glutamate receptor antagonists and NOS inhibitors. These findings suggest that spreading acidification is dependent on extracellular Ca(2+) and glutamate neurotransmission with a contribution from both AMPA and metabotropic glutamate receptors and is modulated by nitric oxide. Therefore, spreading acidification involves both presynaptic and postsynaptic mechanisms. We hypothesize that a regenerative process, i.e., a nonpassive process, is operative that uses the cortical architecture to account for the high speed of propagation.
Figures




Similar articles
-
Nitric oxide-evoked glutamate release and cGMP production in cerebellar slices: control by presynaptic 5-HT1D receptors.Neurochem Int. 2006 Jul;49(1):12-9. doi: 10.1016/j.neuint.2005.12.010. Epub 2006 Feb 13. Neurochem Int. 2006. PMID: 16469416
-
Involvement of kv1 potassium channels in spreading acidification and depression in the cerebellar cortex.J Neurophysiol. 2005 Aug;94(2):1287-98. doi: 10.1152/jn.00224.2005. Epub 2005 Apr 20. J Neurophysiol. 2005. PMID: 15843481
-
Optical imaging of long-term depression in the mouse cerebellar cortex in vivo.J Neurosci. 2003 Mar 1;23(5):1859-66. doi: 10.1523/JNEUROSCI.23-05-01859.2003. J Neurosci. 2003. PMID: 12629190 Free PMC article.
-
Glutamate and Its Receptors as Therapeutic Targets for Migraine.Neurotherapeutics. 2018 Apr;15(2):361-370. doi: 10.1007/s13311-018-0616-5. Neurotherapeutics. 2018. PMID: 29508147 Free PMC article. Review.
-
Optical Imaging of Neuronal Activity in the Cerebellar Cortex Using Neutral Red.In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 11. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 11. PMID: 26844317 Free Books & Documents. Review.
Cited by
-
Neuronal nitric oxide synthase expression in cerebellar mutant mice.Cerebellum. 2004;3(3):141-51. doi: 10.1080/14734220410031927. Cerebellum. 2004. PMID: 15543804 Review.
-
Role of pH in a nitric oxide-dependent increase in cytosolic Cl- in retinal amacrine cells.J Neurophysiol. 2011 Aug;106(2):641-51. doi: 10.1152/jn.00057.2011. Epub 2011 May 18. J Neurophysiol. 2011. PMID: 21593387 Free PMC article.
-
Low-frequency oscillations in the cerebellar cortex of the tottering mouse.J Neurophysiol. 2009 Jan;101(1):234-45. doi: 10.1152/jn.90829.2008. Epub 2008 Nov 5. J Neurophysiol. 2009. PMID: 18987121 Free PMC article.
-
Spreading Depolarization Induces a Transient Potentiation of Excitatory Synaptic Transmission.Neuroscience. 2024 Jul 23;551:323-332. doi: 10.1016/j.neuroscience.2024.05.035. Epub 2024 May 29. Neuroscience. 2024. PMID: 38821241 Free PMC article.
-
Radially expanding transglial calcium waves in the intact cerebellum.Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3496-501. doi: 10.1073/pnas.0809269106. Epub 2009 Feb 11. Proc Natl Acad Sci U S A. 2009. PMID: 19211787 Free PMC article.
References
-
- Aitken PG, Tombaugh GC, Turner DA, Somjen GG. Similar propagation of SD and hypoxic SD-like depolarization in rat hippocampus recorded optically and electrically. J Neurophysiol. 1998;80:1514–1521. - PubMed
-
- Bahar S, Fayuk D, Somjen GG, Aitken PG, Turner DA. Mitochondrial and intrinsic optical signals imaged during hypoxia and spreading depression in rat hippocampal slices. J Neurophysiol. 2000;84:311–324. - PubMed
-
- Batchelor AM, Madge DJ, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre-Purkinje cell pathway in rata cerebellar slices. Neuroscience. 1994;63:911–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
