Functional regeneration in a rat Parkinson's model after intrastriatal grafts of glial cell line-derived neurotrophic factor and transforming growth factor beta1-expressing extra-adrenal chromaffin cells of the Zuckerkandl's organ
- PMID: 11739596
- PMCID: PMC6763029
- DOI: 10.1523/JNEUROSCI.21-24-09888.2001
Functional regeneration in a rat Parkinson's model after intrastriatal grafts of glial cell line-derived neurotrophic factor and transforming growth factor beta1-expressing extra-adrenal chromaffin cells of the Zuckerkandl's organ
Abstract
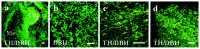
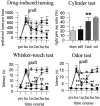
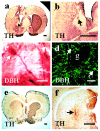
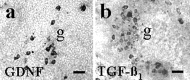
Intrabrain transplantation of chromaffin cell aggregates of the Zuckerkandl's organ, an extra-adrenal paraganglion that has never been tested for antiparkinsonian treatment, induced gradual improvement of functional deficits in parkinsonian rats. These beneficial effects were related to long survival of grafted cells, striatal reinnervation, and enhancement of dopamine levels in grafted striatum. Grafted cells were not dopaminergics, but they expressed glial cell line-derived neurotrophic factor (GDNF) and transforming growth factor-beta(1). These factors were detected in the host striatal tissue, indicating that chromaffin cells secreted them after grafting. Because glial cell line-derived neurotrophic factor possesses neurorestorative properties over dopaminergic neurons, and transforming growth factor-beta(1) is a cofactor that potentiates the neurotrophic actions of GDNF, functional regeneration was likely caused by the chronic trophic action of neurotrophic factors delivered by long-surviving grafted cells. This work should stimulate research on the clinical applicability of transplants of the Zuckerkandl's organ in Parkinson's disease.
Figures
References
-
- Aebischer P, Ridet J-L. Recombinant proteins for neurodegenerative diseases: the delivery issue. Trends Neurosci. 2001;24:533–540. - PubMed
-
- Ahonen M, Soinila S, Joh TH. Pre- and postnatal development of rat retroperitoneal paraganglia. J Auton Nerv Syst. 1987;18:11–120. - PubMed
-
- Bankiewicz KS, Palmatie M, Plunkett RJ, Cummins A, Oldfield EH. Reversal of hemiparkinsonian syndrome in nonhuman primates by amnion implantation into caudate nucleus. J Neurosurg. 1994;81:869–876. - PubMed
-
- Batchelor PE, Liberatore GT, Wong JYF, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. - PMC - PubMed
-
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
