Adaptation to temporal contrast in primate and salamander retina
- PMID: 11739598
- PMCID: PMC6763043
- DOI: 10.1523/JNEUROSCI.21-24-09904.2001
Adaptation to temporal contrast in primate and salamander retina
Abstract
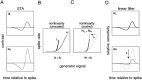
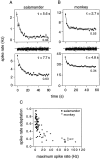
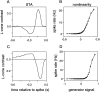
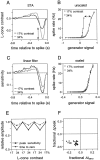
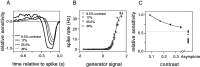
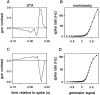
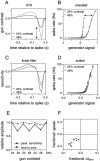
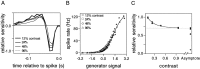
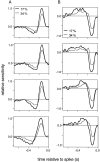
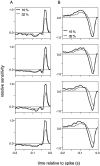
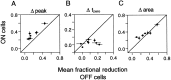
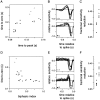
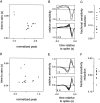
Visual adaptation to temporal contrast (intensity modulation of a spatially uniform, randomly flickering stimulus) was examined in simultaneously recorded ensembles of retinal ganglion cells (RGCs) in tiger salamander and macaque monkey retina. Slow contrast adaptation similar to that recently discovered in salamander and rabbit retina was observed in monkey retina. A novel method was developed to quantify the effect of temporal contrast on steady-state sensitivity and kinetics of light responses, separately from nonlinearities that would otherwise significantly contaminate estimates of sensitivity. Increases in stimulus contrast progressively and reversibly attenuated and sped light responses in both salamander and monkey RGCs, indicating that a portion of the contrast adaptation observed in visual cortex originates in the retina. The effect of adaptation on sensitivity and kinetics differed in simultaneously recorded populations of ON and OFF cells. In salamander, adaptation affected the sensitivity of OFF cells more than ON cells. In monkey, adaptation affected the sensitivity of ON cells more than OFF cells. In both species, adaptation sped the light responses of OFF cells more than ON cells. Functionally defined subclasses of ON and OFF cells also exhibited asymmetric adaptation. These findings indicate that contrast adaptation differs in parallel retinal circuits that convey distinct visual signals to the brain.
Figures

References
-
- Anstis S. Adaptation to peripheral flicker. Vision Res. 1996;36:3479–3485. - PubMed
-
- Baccus S, Meister M. Mechanisms of contrast adaptation in the retina. Soc Neurosci Abstr. 2000;26:1328.
-
- Benardete EA, Kaplan E. The dynamics of primate m retinal ganglion cells. Vis Neurosci. 1999;16:355–368. - PubMed
-
- Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Vis Neurosci. 1992;8:483–486. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
