Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251
- PMID: 11739667
- PMCID: PMC135704
- DOI: 10.1128/jvi.76.1.9-18.2002
Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251
Abstract
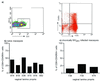
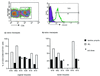
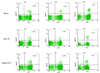
Most human immunodeficiency virus (HIV) type 1 infections occur by the mucosal route. Thus, it is important to assess the immune responses to HIV in the vaginal, cervical, and rectal compartments. Here we quantitated the virus-specific CD8+ T-cell response and characterized the phenotype of lymphocytes in the genital tracts of naive macaques, macaques acutely or chronically infected with simian immunodeficiency virus SIVmac251, and macaques chronically infected with chimeric simian/human immunodeficiency virus SHIV(KU2.) Vaginal biopsy samples or samples obtained at the time of euthanasia were used in this analysis. The percentage of Gag-specific, tetramer-positive T cells was as high as 13 to 14% of the CD3+ CD8+ T-cell population in the vaginal and cervical laminae propriae of both SIVmac251 and SHIV(KU2) chronically infected macaques. In most cases, the frequency of this response in the cervicovaginal compartment far exceeded the frequency in the blood or the draining iliac lymph node. Vaginal laminae propriae of naive macaques contained 55 to 65% CD3+ CD8+ cells and 28 to 34% CD3+ CD4+ cells, while the majority of intraepithelial cells were CD8+ T cells (75 to 85%). For the same cells, the surface expression of CD62L was low whereas that of alphaEbeta7 was high. No difference in the expression of CD45RA on CD8+ T cells was observed in the chronic stage of SIVmac251 infection. Although no decrease in the percentage of CD4+ cells in the genital tract was observed within the first 12 days of infection, by 6 weeks from SIVmac251 infection and thereafter the percentage of CD4+ T cells was decreased in the laminae propriae of the vagina and cervix. Expression of CD45RA did not differ in naive and acutely SIVmac251 infected macaques. Information on the quality and quantity of local immune responses may help in the design of vaccine strategies aimed at containing viral replication at the site of viral encounter.
Figures



References
-
- Allen, T. M., D. H. O’Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386–390. - PubMed
-
- Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94–96. - PubMed
-
- Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95:1709–1714. - PMC - PubMed
-
- Beyrer, C., A. W. Artenstein, S. Rugpao, H. Stephens, T. C. VanCott, M. L. Robb, M. Rinkaew, D. L. Birx, C. Khamboonruang, P. A. Zimmerman, K. E. Nelson, and C. Natpratan. 1999. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. Chiang Mai HEPS Working Group. J. Infect. Dis. 179:59–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

