Binding of LFA-1 (CD11a) to intercellular adhesion molecule 3 (ICAM-3; CD50) and ICAM-2 (CD102) triggers transmigration of human immunodeficiency virus type 1-infected monocytes through mucosal epithelial cells
- PMID: 11739669
- PMCID: PMC135694
- DOI: 10.1128/jvi.76.1.32-40.2002
Binding of LFA-1 (CD11a) to intercellular adhesion molecule 3 (ICAM-3; CD50) and ICAM-2 (CD102) triggers transmigration of human immunodeficiency virus type 1-infected monocytes through mucosal epithelial cells
Abstract
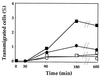
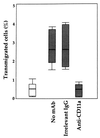
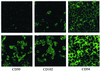
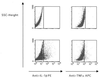
Transmigration of human immunodeficiency virus (HIV)-infected mononuclear cells through the genital mucosa is one of the possible mechanisms of sexual transmission of HIV. Here, we investigated the transmigration of cell-associated R5-tropic HIV type 1 (HIV-1) through a tight monolayer of human epithelial cells in vitro. We show that this process is dependent on an initial interaction between alphaLbeta2 integrin CD11a/CD18 on infected monocytic cells and intercellular adhesion molecule 2 (ICAM-2; CD102) and ICAM-3 (CD50) on the apical membrane of epithelial cells. The CD50 and CD102 ligands were overexpressed on epithelial cells when the cells were activated by proinflammatory cytokines in the cellular microenvironment. An accumulation of proviral DNA was found in the transmigrated cells, clearly reflecting the preferential transepithelial migration of HIV-1-infected cells under proinflammatory conditions. Our observations provide new insights supporting the hypothesis that HIV-infected mononuclear cells contained in genital secretions from infected individuals may cross the epithelial genital mucosa of an exposed receptive sexual partner, particularly under inflammatory conditions of damaged genital tissue. Understanding the fundamental aspects of the initial HIV entry process during sexual transmission remains a critical step for preventing human infection and developing further vaccinal strategies and virucidal agents.
Figures





References
-
- Amzazi, S., L. Ylisastigui, Y. Bakri, L. Rabehi, L. Gattegno, M. Parmentier, J. Gluckman, and A. Benjouad. 1998. The inhibitory effect of RANTES on the infection of primary macrophages by R5 human immunodeficiency virus-type 1 depends on the macrophage activation state. Virology 252:96–105. - PubMed
-
- Ball, J. M., Z. Moldoveanu, L. R. Melsen, P. A. Kozlowski, S. Jackson, M. J. Mulligan, J. F. Mestecky, and R. W. Compans. 1995. A polarized human endometrial cell line that binds and transports polymeric IgA. In Vitro Cell. Dev. Biol. Anim. 31:196–206. - PubMed
-
- Belec, L., R. Gherardi, C. Payan, T. Prazuck, J.-E. Malkin, C. Tévi-Bénissan, and J. Pillot. 1995. Proinflammatory cytokine expression in cervicovaginal secretions of normal and HIV-infected women. Cytokine 7:568–574. - PubMed
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657–700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

