Vaccine-induced immune responses in rodents and nonhuman primates by use of a humanized human immunodeficiency virus type 1 pol gene
- PMID: 11739684
- PMCID: PMC135696
- DOI: 10.1128/jvi.76.1.185-194.2002
Vaccine-induced immune responses in rodents and nonhuman primates by use of a humanized human immunodeficiency virus type 1 pol gene
Abstract
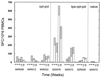
A synthetic gene consisting of the reverse transcriptase (RT) and integrase (IN) domains of human immunodeficiency virus type 1 (HIV-1) pol was constructed using codons most frequently used in humans. The humanized pol gave dramatically improved levels of Rev-independent, in vitro protein production in mammalian cells and elicited much stronger cellular immunity in rodents than did virus-derived gene. Specifically, BALB/c mice were immunized with plasmids and/or recombinant vaccinia virus constructs expressing the synthetic gene. High frequencies of Pol-specific T lymphocytes were detected in these animals by the gamma interferon enzyme-linked immunospot assay against pools of short overlapping peptides. Characterization of the stimulatory peptides from these pools indicates that the optimized gene constructs are able to effectively activate both CD4+ and CD8+ T cells. Immunization of rhesus macaques with DNA vaccines expressing the humanized pol coupled to a human tissue plasminogen activator leader sequence led to pronounced in vitro cytotoxic T-lymphocyte killing activities and enhanced levels of circulating Pol-specific T cells, comparable to those observed in HIV-1-infected human subjects. Thus, optimizing the immunogenic properties of HIV-1 Pol at the level of the gene sequence validates it as an antigen and provides an important step toward the construction of a potent pol-based HIV-1 vaccine component.
Figures





References
-
- Bagarazzi, M. L., J. D. Boyer, M. A. Javadian, M. A. Chattergoon, A. R. Shah, A. D. Cohen, M. K. Bennett, R. B. Ciccarelli, K. E. Ugen, and D. B. Weiner. 1999. Systemic and mucosal immunity is elicited after both intramuscular and intravaginal delivery of human immunodeficiency virus type 1 DNA plasmid vaccines to pregnant chimpanzees. J. Infect. Dis. 180:1351–1355. - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. Trigona, K. Punt, L. Guan, S. Dubey, D. Casimiro, A. Simon, M.-E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486–492. - PubMed
-
- Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Crangae, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170–4182. - PMC - PubMed
-
- Borg, J.-P., H.-G. Ihlenfeldt, G. Jung, G. Haas, and M. Pierres. 1994. Human immunodeficiency virus-1 reverse transcriptase immunodominant CD4+ T cell epitopes: a peptide-based multiparametric assessment in the mouse. Eur. J. Immunol. 24:1496–1502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

