Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice
- PMID: 11739689
- PMCID: PMC135692
- DOI: 10.1128/jvi.76.1.243-250.2002
Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice
Abstract
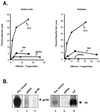
The effects of genetic adjuvants on humoral and cell-mediated immunity to two human immunodeficiency virus antigens, Env and Nef, have been examined in mice. Despite similar levels of gene expression and the same gene delivery vector, the immune responses to these two gene products differed following DNA immunization. Intramuscular immunization with a Nef expression vector plasmid generated a humoral response and antigen-specific gamma interferon (IFN-gamma) production but little cytotoxic-T-lymphocyte (CTL) immunity. In contrast, immunization with an Env vector stimulated CTL activity but did not induce a high-titer antibody response. The ability to modify these antigen-specific immune responses was investigated by coinjection of DNA plasmids encoding cytokine and/or hematopoietic growth factors, interleukin-2 (IL-2), IL-12, IL-15, Flt3 ligand (FL), and granulocyte-macrophage colony-stimulating factor (GM-CSF). Coadministration of these genes largely altered the immune responses quantitatively but not qualitatively. IL-12 induced the greatest increase in IFN-gamma and immunoglobulin G responses to Nef, and GM-CSF induced the strongest IFN-gamma and CTL responses to Env. A dual approach of expanding innate immunity by administering the FL gene, together with a cytokine that enhances adaptive immune responses, IL-2, IL-12, or IL-15, generated the most potent immune response at the lowest doses of Nef antigen. These findings suggest that intrinsic properties of the antigen determine the character of immune reactivity for this method of immunization and that specific combination of innate and adaptive immune cytokine genes can increase the magnitude of the response to DNA vaccines.
Figures



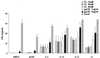

Similar articles
-
Improving recombinant MVA immune responses: potentiation of the immune responses to HIV-1 with MVA and DNA vectors expressing Env and the cytokines IL-12 and IFN-gamma.Virus Res. 2006 Mar;116(1-2):11-20. doi: 10.1016/j.virusres.2005.08.008. Epub 2005 Oct 7. Virus Res. 2006. PMID: 16214252
-
Modulation of cellular and humoral immune responses to a multiepitopic HIV-1 DNA vaccine by interleukin-18 DNA immunization/viral protein boost.Vaccine. 2001 Apr 6;19(20-22):2803-11. doi: 10.1016/s0264-410x(01)00004-4. Vaccine. 2001. PMID: 11282190
-
ELISPOT analysis of a new CTL based DNA vaccine for HIV-1 using GM-CSF in DNA prime/peptide boost strategy: GM-CSF induced long-lived memory responses.Immunol Lett. 2011 Oct 30;140(1-2):14-20. doi: 10.1016/j.imlet.2011.05.005. Epub 2011 May 23. Immunol Lett. 2011. PMID: 21679728
-
DNA mucosal HIV vaccine in humans.Virus Res. 2002 Jan 30;82(1-2):141-5. doi: 10.1016/s0168-1702(01)00396-3. Virus Res. 2002. PMID: 11885941 Review.
-
DNA vaccine strategies: candidates for immune modulation and immunization regimens.Methods. 2003 Nov;31(3):207-16. doi: 10.1016/s1046-2023(03)00135-x. Methods. 2003. PMID: 14511953 Review.
Cited by
-
Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity.Proc Natl Acad Sci U S A. 2003 Mar 18;100(6):3392-7. doi: 10.1073/pnas.0630592100. Epub 2003 Mar 7. Proc Natl Acad Sci U S A. 2003. PMID: 12626740 Free PMC article.
-
Physical trauma of vaccination acts as a wake-up call to dangers in the skin.Immunology. 2003 Nov;110(3):291-2. doi: 10.1046/j.1365-2567.2003.01740.x. Immunology. 2003. PMID: 14632654 Free PMC article. Review. No abstract available.
-
Therapeutic immunization for HIV.Springer Semin Immunopathol. 2006 Nov;28(3):221-30. doi: 10.1007/s00281-006-0029-0. Epub 2006 Oct 10. Springer Semin Immunopathol. 2006. PMID: 17031650 Review.
-
Induction of specific immune responses by severe acute respiratory syndrome coronavirus spike DNA vaccine with or without interleukin-2 immunization using different vaccination routes in mice.Clin Vaccine Immunol. 2007 Jul;14(7):894-901. doi: 10.1128/CVI.00019-07. Epub 2007 May 9. Clin Vaccine Immunol. 2007. PMID: 17494640 Free PMC article.
-
Naloxone/alum mixture a potent adjuvant for HIV-1 vaccine: induction of cellular and poly-isotypic humoral immune responses.Pathog Glob Health. 2016 Mar;110(2):39-47. doi: 10.1179/2047773215Y.0000000035. Epub 2016 Apr 13. Pathog Glob Health. 2016. PMID: 26403975 Free PMC article.
References
-
- Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526–5540. - PMC - PubMed
-
- Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 97:4192–4197. - PMC - PubMed
-
- Barouch, D. H., S. Santra, T. D. Steenbeke, X. X. Zheng, H. C. Perry, M. E. Davies, D. C. Freed, A. Craiu, T. B. Strom, J. W. Shiver, and N. L. Letvin. 1998. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J. Immunol. 161:1875–1882. - PubMed
-
- Boyer, J. D., J. Kim, K. E. Ugen, A. D. Cohen, L. Ahn, K. Schumann, K. Lacy, M. L. Bagarazzi, A. Javadian, R. Ciccarelli, R. S. Ginsberg, R. R. MacGregor, and D. B. Weiner. 1999. HIV-1 DNA vaccines and chemokines. Vaccines 17(Suppl. 2):53–64. - PubMed
-
- Cho, J. H., S. W. Lee, and Y. C. Sung. 1999. Enhanced cellular immunity to hepatitis C virus nonstructural proteins by codelivery of granulocyte macrophage-colony stimulating factor gene in intramuscular DNA immunization. Vaccine 17:1136–1144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

