Cytoplasmic death signal triggered by SRC-mediated phosphorylation of the adenovirus E4orf4 protein
- PMID: 11739721
- PMCID: PMC134208
- DOI: 10.1128/MCB.22.1.41-56.2002
Cytoplasmic death signal triggered by SRC-mediated phosphorylation of the adenovirus E4orf4 protein
Abstract
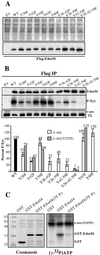
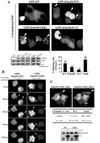
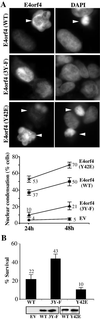
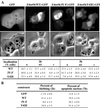
In transformed cells, the adenovirus E4orf4 death factor works in part by inducing a Src-mediated cytoplasmic apoptotic signal leading to caspase-independent membrane blebbing and cell death. Here we show that Src-family kinases modulate E4orf4 phosphorylation on tyrosine residues. Mutation of tyrosines 26, 42, and 59 to phenylalanines inhibited Src-induced phosphorylation of E4orf4 in vivo and in vitro but had no effect on the molecular association of E4orf4 with Src. However, in contrast to wild-type E4orf4, the nonphosphorylatable E4orf4 mutant was unable to modulate Src-dependent phosphorylation and was deficient in recruiting a subset of tyrosine-phosphorylated proteins. Indeed, the Src substrates cortactin and p62dok were found to associate with wild-type E4orf4 but not with the nonphosphorylatable E4orf4. Importantly, the nonphosphorylatable mutant E4orf4 was preferentially distributed in the cell nucleus, was unable to induce membrane blebbing, and had a highly impaired killing activity. Conversely, an activated form of E4orf4 was obtained by mutation of tyrosine 42 to glutamic acid. This pseudophosphorylated mutant E4orf4 was enriched in the cytoplasm and plasma membrane, showed increased binding to phosphotyrosine-containing proteins, and induced a dramatic blebbing phenotype associated with increased cell death. Altogether, our findings strongly suggest that Src-mediated phosphorylation of adenovirus type 2 E4orf4 is critical to promoting its cytoplasmic and membrane localization and is required for the transduction of E4orf4-Src-dependent induction of membrane blebbing. We propose that E4orf4 acts in part by uncoupling Src-dependent signals to drive the formation of a signaling complex that triggers a cytoplasmic death signal.
Figures





Similar articles
-
Adenovirus E4 open reading frame 4-induced apoptosis involves dysregulation of Src family kinases.J Cell Biol. 2000 Sep 4;150(5):1037-56. doi: 10.1083/jcb.150.5.1037. J Cell Biol. 2000. PMID: 10973994 Free PMC article.
-
Distinct cell death pathways triggered by the adenovirus early region 4 ORF 4 protein.J Cell Biol. 2002 Aug 5;158(3):519-28. doi: 10.1083/jcb.200201106. Epub 2002 Aug 5. J Cell Biol. 2002. PMID: 12163473 Free PMC article.
-
Activation of adenovirus type 2 early region 4 ORF4 cytoplasmic death function by direct binding to Src kinase domain.J Biol Chem. 2004 Jun 11;279(24):25905-15. doi: 10.1074/jbc.M400933200. Epub 2004 Apr 7. J Biol Chem. 2004. PMID: 15070897
-
Mechanisms of cancer cell killing by the adenovirus E4orf4 protein.Viruses. 2015 May 7;7(5):2334-57. doi: 10.3390/v7052334. Viruses. 2015. PMID: 25961489 Free PMC article. Review.
-
Induction of transformed cell-specific apoptosis by the adenovirus E4orf4 protein.Prog Mol Subcell Biol. 2004;36:245-67. doi: 10.1007/978-3-540-74264-7_12. Prog Mol Subcell Biol. 2004. PMID: 15171615 Review. No abstract available.
Cited by
-
The adenovirus E4orf4 protein targets PP2A to the ACF chromatin-remodeling factor and induces cell death through regulation of SNF2h-containing complexes.Nucleic Acids Res. 2011 Aug;39(15):6414-27. doi: 10.1093/nar/gkr231. Epub 2011 May 5. Nucleic Acids Res. 2011. PMID: 21546548 Free PMC article.
-
The adenoviral protein E4orf4: a probing tool to decipher mechanical stress-induced nuclear envelope remodeling in tumor cells.Cell Cycle. 2020 Nov;19(22):2963-2981. doi: 10.1080/15384101.2020.1836441. Epub 2020 Oct 25. Cell Cycle. 2020. PMID: 33103553 Free PMC article.
-
JNK-mediated phosphorylation of paxillin in adhesion assembly and tension-induced cell death by the adenovirus death factor E4orf4.J Biol Chem. 2008 Dec 5;283(49):34352-64. doi: 10.1074/jbc.M803364200. Epub 2008 Sep 25. J Biol Chem. 2008. PMID: 18818208 Free PMC article.
-
Regulation of cell death by recycling endosomes and golgi membrane dynamics via a pathway involving Src-family kinases, Cdc42 and Rab11a.Mol Biol Cell. 2009 Sep;20(18):4091-106. doi: 10.1091/mbc.e09-01-0057. Epub 2009 Jul 29. Mol Biol Cell. 2009. PMID: 19641023 Free PMC article.
-
Adenovirus E4orf4 hijacks rho GTPase-dependent actin dynamics to kill cells: a role for endosome-associated actin assembly.Mol Biol Cell. 2006 Jul;17(7):3329-44. doi: 10.1091/mbc.e05-12-1146. Epub 2006 May 10. Mol Biol Cell. 2006. PMID: 16687574 Free PMC article.
References
-
- Abram, C. L., and S. A. Courtneidge. 2000. Src family tyrosine kinases and growth factor signaling. Exp. Cell Res. 254:1–13. - PubMed
-
- Alexandropoulos, K., and D. Baltimore. 1996. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 10:1341–1355. - PubMed
-
- Ashkenazi, A., and V. M. Dixit. 1999. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11:255–260. - PubMed
-
- Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305–1308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
