Protection from free beta-tubulin by the beta-tubulin binding protein Rbl2p
- PMID: 11739729
- PMCID: PMC134216
- DOI: 10.1128/MCB.22.1.138-147.2002
Protection from free beta-tubulin by the beta-tubulin binding protein Rbl2p
Abstract
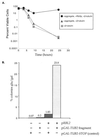
Free beta-tubulin not in heterodimers with alpha-tubulin can be toxic, disrupting microtubule assembly and function. We are interested in the mechanisms by which cells protect themselves from free beta-tubulin. This study focused specifically on the function of Rbl2p, which, like alpha-tubulin, can rescue cells from free beta-tubulin. In vitro studies of the mammalian homolog of Rbl2p, cofactor A, have suggested that Rbl2p/cofactor A may be involved in tubulin folding. Here we show that Rbl2p becomes essential in cells containing a modest excess of beta-tubulin relative to alpha-tubulin. However, this essential activity of Rbl2p/cofactorA does not depend upon the reactions described by the in vitro assay. Rescue of beta-tubulin toxicity requires a minimal but substoichiometric ratio of Rbl2p to beta-tubulin. The data suggest that Rbl2p binds transiently to free beta-tubulin, which then passes into an aggregated form that is not toxic.
Figures




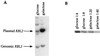

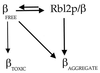
References
-
- Archer, J. E. 1996. PhD. thesis. Massachusetts Institute of Technology, Cambridge.
-
- Archer, J. E., L. R. Vega, and F. Solomon. 1995. Rbl2p, a yeast protein that binds to β-tubulin and participates in microtubule function in vivo. Cell 82:425–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
