Absence of the CAAX endoprotease Rce1: effects on cell growth and transformation
- PMID: 11739732
- PMCID: PMC134215
- DOI: 10.1128/MCB.22.1.171-181.2002
Absence of the CAAX endoprotease Rce1: effects on cell growth and transformation
Abstract
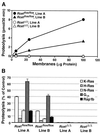
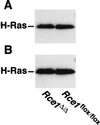
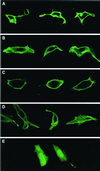
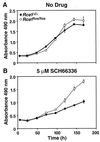
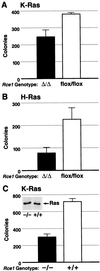
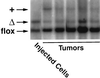
After isoprenylation, the Ras proteins and other CAAX proteins undergo two additional enzymatic modifications-endoproteolytic release of the last three amino acids of the protein by the protease Rce1 and methylation of the carboxyl-terminal isoprenylcysteine by the methyltransferase Icmt. This postisoprenylation processing is thought to be important for the association of Ras proteins with membranes. Blocking postisoprenylation processing, by inhibiting Rce1, has been suggested as a potential approach for retarding cell growth and blocking cellular transformation. The objective of this study was to develop a cell culture system for addressing these issues. We generated mice with a conditional Rce1 allele (Rce1(flox)) and produced Rce1(flox/flox) fibroblasts. Cre-mediated excision of Rce1 (thereby producing Rce1(Delta/Delta) fibroblasts) eliminated Ras endoproteolytic processing and methylation and caused a partial mislocalization of truncated K-Ras and H-Ras fusion proteins within cells. Rce1(Delta/Delta) fibroblasts grew more slowly than Rce1(flox/flox) fibroblasts. The excision of Rce1 also reduced Ras-induced transformation, as judged by the growth of colonies in soft agar. The excision of Rce1 from a Rce1(flox/flox) skin carcinoma cell line also significantly retarded the growth of cells, and this effect was exaggerated by cotreatment of the cells with a farnesyltransferase inhibitor. These studies support the idea that interference with postisoprenylation processing retards cell growth, limits Ras-induced transformation, and sensitizes tumor cells to a farnesyltransferase inhibitor.
Figures


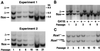


References
-
- Ashby, M. N., and J. Rine. 1995. Ras and a-factor converting enzyme. Methods Enzymol. 250:235–251. - PubMed
-
- Bergo, M. O., G. K. Leung, P. Ambroziak, J. C. Otto, P. J. Casey, and S. G. Young. 2000. Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J. Biol. Chem. 275:17605–17610. - PubMed
-
- Boyartchuk, V. L., M. N. Ashby, and J. Rine. 1997. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275:1796–1800. - PubMed
-
- Brown, M. S., and J. L. Goldstein. 1993. Protein prenylation. Mad bet for Rab. Nature 366:14–15. - PubMed
-
- Chakravarti, D., P. Mailander, J. Franzen, S. Higginbotham, E. L. Cavalieri, and E. G. Rogan. 1998. Detection of dibenzol[a,l]pyrene-induced H-ras codon 61 mutant genes in preneoplastic SENCAR mouse skin using a new PCR-RFLP method. Oncogene 16:3203–3210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
